PRELIMINARY NOTE
On March 15, 2012 we were invited to participate in the Madrid meeting to debate some of the aspects related to the paper on activation of vitamin D receptor (VDR) activators in the optimisation of secondary hyperparathyroidism (SHPT) on dialysis. There were clearly diverse opinions among the participants drawn from their own experience in managing SHPT, from the traditional approach with VDR activators (VDRA) or with selective VDR activators (sVDRA) for some and with calcimimetics (cinacalcet) for others. Obviously, the positions of experts are summarised in the recommendations of the Spanish Nephrology Society for the management of alterations in bone-mineral metabolism in patients with chronic kidney disease (CKD),1 so the points we present here are only those that were discussed in this meeting and the consensus reached by all the signatories.
Vitamin D deficiency is a known risk factor, among the pathogenic factors responsible for the development of SHPT during CKD. The toxicity of active vitamin D metabolite drugs, especially calcitriol, is primarily due to increases in calcium and phosphorus levels. This has led to the need to develop vitamin D analogues with less effect on a positive calcium and phosphorus balance.2-6 Using different mechanisms, the contribution of calcimimetics, which are sometimes used concomitantly because of their complementary effect on vitamin D, has led to better control of SHPT.
The objective of this meeting was to discuss the scientific evidence of the different treatments involved in the management of SHPT in order to optimise the treatment of dialysis patients based on their efficacy, safety and cost.
HISTORY
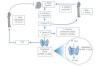
SHPT is a common and severe consequence of CKD (Figure 1). This disorder has a complex pathogenesis primarily characterised by a decrease in 1,25-dihydroxyvitamin D or calcitriol (with the resulting deficit in activation of its receptor) and a decrease in phosphaturia (despite a decrease in tubular phosphorus reabsorption and due to a decrease in the filtered load), anomalies in serum calcium and phosphorus levels, parathyroid gland hyperplasia, increased parathyroid hormone (PTH) secretion and systemic bone and mineral anomalies.7
Inadequate control of SHPT and bone mineral disorder can lead to vascular calcification and contribute to an increase in cardiovascular mortality, which is the most common cause of death in dialysis patients.
There are two classically-known receptors in the parathyroid glands, the VDR and the calcium-sensing receptor (CaSR). These modulate its function. Activation of these receptors causes a decrease in PTH secretion and inhibition of cell proliferation.
At present, we have available three classes of drugs for the treatment of SHPT:
Several published studies have shown that the three options are effective in suppressing PTH. However, clinical studies indicate that non-selective VDRAs can lead to dose-limited hyperphosphataemia and hypercalcaemia. The sVDRAs have less effect on intestinal calcium absorption and calcium and phosphorus mobilization in bone, leading to fewer episodes of hypercalcaemia and hyperphosphatemia.8 Calcimimetics are also very effective, although they are often associated with hypocalcaemia.9
One of the current problems in defining SHPT is the diversity of procedures for measuring PTH. Because of this, different guidelines refer to different normal ranges for PTH in dialysis patients. Nevertheless, as recognized by the KDIGO guidelines, clinical judgment in SHPT should be based not only on the absolute PTH level within the normal range, but also on the progressive tendency for an increase in levels. The rational histological basis for considering this tendency is progressive transformation of parathyroid glands, which develop nodular hyperplasia with a decrease in VDR and calcium receptors, thereby leading to progressive resistance in SHPT to different treatment options (VDRA and calcimimetics).10
SELECTIVE (II) AND NON-SELECTIVE VITAMIN D RECEPTOR ACTIVATORS
The VDR in the parathyroid glands is a cytosolic receptor that acts as a transcription factor in PTH expression. Once the VDR-activator complex has been activated, it acts on the vitamin D response element in the parathyroid gland. This causes a decrease in the production of PTH RNA. Binding to the response element is conditioned by the action co-activating and/or co-repressor elements. As a result, the progressive decrease in serum 1,25-dihydroxyvitamin D levels in CKD causes an increase in PTH synthesis in the parathyroid glands. The absence of this inhibitory stimulus through the VDR leads to a decrease in the receptor population and causes hyperplasia of the parathyroid gland. This hyperplasia occurs in order to satisfy the demand for PTH secretion. This gland, which has few receptors, ends up becoming an autonomous gland that is incapable of responding to treatment and sometimes requires surgery.
The distribution of VDRs is practically universal, as are the multiple genes regulated by its activation. It plays an essential role in cardiovascular health. In patients with CKD, VDR activation may have beneficial effects, not only on mineral metabolism but also on the kidney disease itself.11 It not only inhibits inflammatory markers, but also modulates the immune response and induces regulation to decrease renin. The VDR is also important in stimulating remodelling of cardiac muscle.11
There are a large number of molecules in the “vitamin D and analogues” therapeutic group. This includes calcitriol (the most active form of vitamin D) and its precursors such as 25-OH vitamin D, calcitriol pro-hormones that are ultimately transformed into 1,25-dihydroxyvitamin D or calcitriol by one pathway or another. Therefore, this group of drugs makes up the non-selective VDRA group since they act on the body in the same manner as endogenous vitamin D. One of the main problems associated with non-selective VDRAs is the increase in blood calcium and phosphorus levels and the complex management involved in administering them, which leads to limited dosages. The new selective activators have shown similar or superior dose-equivalence in clinical trials for suppressing PTH with less calcaemic and hyperphosphataemic activity.12 They demonstrated a faster reduction in PTH compared to patients treated with paricalcitol with fewer episodes of hypercalcaemia and/or increase in the calcium x phosphorus product (10% vs. 38%, P=.008).13 This increase in calcium and phosphorus is associated with vascular calcification, which would provoke an increase in morbidity and mortality in dialysis patients.14
Many patients with CKD cannot receive adequate treatment for SHPT with active vitamin D due to their risks for hypercalcaemia, hypercalciuria, hyperphosphataemia and an increase in the calcium x phosphorus product.5,15,16 In addition, other options for controlling SHPT in CKD patients who are not in dialysis such as calcimimetics are not recommended since they cause hypercalciuria, hypocalcaemia and increased serum phosphorus.17,18
Because of their chemical structures, selective and non-selective VDR activators have heterogeneous genomic effects that initiate different responses in the parathyroid gland, intestine and bone. Selective VDR activators have less effect on intestinal calcium absorption and calcium and phosphorus mobilisation in bone compared to non-selective VDRAs. The use of specific VDR activators will help combat the undesirable effects of hypercalcaemia and hyperphosphataemia. We will see that there are clinical data that suggest an association between VDRAs and an increase in CKD patient survival.19-25
Effect on survival
There are studies that suggest that VDRAs are associated with greater survival in patients with CKD on haemodialysis.24 This benefit is greater with selective than with non-selective VDRAs.26
In a historic cohort study, Teng et al. compared the 36-month survival rates with paricalcitol and calcitriol in nearly 68 000 dialysis patients.24 The results showed a significant improvement in survival in patients who received paricalcitol which was already seen at 12 months and increased over time. The results were the same when survival was analysed in patients who switched from one treatment to another. The mortality rate was 16% lower in patients treated with paricalcitol. Kalantar et al. (2006), in a new historic cohort study, evaluated survival in 58 000 patients, demonstrating that any dosage of paricalcitol that was administered was associated with a benefit in survival (reduction of all-cause mortality or cardiovascular mortality), versus patients who did not receive paricalcitol.26 Lee et al. (2007), in an extension of the Kalantar et al. study that included new data from a subgroup analysis based on race, presence or not of diabetes, sex, age, time on dialysis, serum albumin levels, protein intake, calcium and phosphorus levels, calcium x phosphorus product, alkaline phosphatase and PTH, observed an increase in survival for all subgroups treated with paricalcitol versus untreated patients in each of the studied strata.27 The results from the FARO study conducted by the Italian haemodialysis group were recently published. These results corroborate the conclusions of the studies by Kalantar et al. and Teng et al., that VDR activation increases survival in the group of patients treated with paricalcitol versus patients treated with calcitriol with a statistically significant difference. Tentori et al. obtained similar results in another study in which they compared all-cause deaths and cardiovascular deaths in haemodialysis patients treated with VDRAs (calcitriol, doxercalciferol and paricalcitol), demonstrating that mortality was similar in the paricalcitol and doxercalciferol groups and greater in the calcitriol group (P<.001).29 Vervloet et al. analysed the different observational studies which associated the relationship between VDRA use and mortality. They maintained that, although all of the data that considered effects on mortality with VDRA therapy in haemodialysis patients came from historic cohorts, the analysis should be considered valid for clinical practice since the five existing observational studies used solid methodologies.30
Therefore, the use of VDRAs is associated with a benefit in survival in CKD patients, regardless of the effects on PTH, calcium and phosphorus, selective VDR activation with paricalcitol appears better compared to non-activation or activation with other molecules such as calcitriol. The mechanisms which associate survival and cardiovascular benefits to VDR activation are still being researched, but different factors may play an important role now that the VDR has been identified in 30 different tissues in the human body.31
Control of secondary hyperparathyroidism and bone mineral metabolism.
As we will see later on, selective activators have less hypercalcaemia- and hyperphosphataemia-causing effects than calcitriol. Several studies have confirmed their ability to achieve a reduction in PTH levels more quickly and for a sustained period of time than that which is produced by non-selective VDRAs, as shown in Table 1. In 2001, Llach et al., in a prospective study on 37 patients who were resistant to calcitriol treatment (PTH>600), demonstrated that after 16 months of treatment with paricalcitol in a 1:3 or 1:4 conversion ratio, there was a significant reduction in PTH with no statistically significant changes in calcium and phosphorus levels.32 Afterwards, Sprage et al., in a 2003 study in which 263 dialysis patients participated, demonstrated that the time to reach the normal levels in the KDOQI guidelines was in week 18 in the paricalcitol group and at no time in the calcitriol group after 32 weeks of medical treatment. They also showed that there were less episodes of hypercalcaemia or increases in the calcium phosphorus product compared to patients treated with calcitriol (P=.008).13
In 2009, Abdul Gafor et al., in a single-centre study on 25 patients, demonstrated a significant decrease in PTH in the paricalcitol group, increasing calcium levels almost exclusively in the calcitriol group.8
In a 2010 study in 59 patients treated with calcitriol for at least 12 months who then completed another 12 months with paricalcitol, Mittman et al. demonstrated that switching from calcitriol to paricalcitol revealed a decrease in calcium, phosphorus, calcium x phosphorus product and PTH in addition to reducing alkaline phosphatase. In addition, they demonstrated a very significant difference in the number of dose losses during treatment in favour of paricalcitol.33
Finally, Tonbul et al. published a study that year (with a similar design to Llach et al.) in 43 patients who were refractory to calcitriol treatment. At the end of the study, the results revealed a decrease in PTH, maintaining an increase in serum phosphorus and an increase (though not statistically significant) in serum calcium.34
Intestinal calcium absorption
As stated previously, one of the great problems with VDRAs is the increase in calcium and phosphorus levels primarily due to their intestinal absorption and mobilisation from the bone. Unlike treatment with paricalcitol, calcitriol treatment has been associated with an increase in the number of intestinal VDRs. In addition, a ten-time greater dosage of calcitriol is needed to produce similar increases in serum calcium and phosphorus levels while at the same time needing a three-times greater dosage in order to maintain the same effect on PTH, which offers a greater therapeutic window with paricalcitol.35
Takahashi et al. (1997) demonstrated that uraemic rats treated with paricalcitol expressed less VDRs in the membranes of intestinal cells than uraemic rats treated with calcitriol after 8 weeks of treatment.35 Afterwards, in 2002, Brown et al. demonstrated that paricalcitol decreases the expression of the primary calcium transporter proteins in intestinal cells (calbindin D, CaT1 calcium channel and the PMCA1 calcium pump) in an experimental model versus calcitriol, providing a greater increase in intestinal calcium absorption.36 Years later, Nakane et al.37 corroborated that paricalcitol was associated with less calcium absorption in uraemic rats which were fed a phosphorus-rich diet for 12 days, with less expression of transporter proteins in the paricalcitol group.37 Lund et al., in a clinical crossover study in 29 haemodialysis patients, demonstrated that the fractioned absorption of intestinal calcium was significantly lower after treatment with paricalcitol (0.135±0.022), with 0.023 being the absolute difference in fractioned calcium absorption. There were no significant differences in PTH, calcium, phosphorus or the calcium x phosphorus product.38 Very recently, Martinez et al. demonstrated for the first time that there was less urine calcium secretion in patients treated with paricalcitol versus those treated with calcitriol, with the difference being statistically significant (P=.0347).39
Action in bone
Different studies have shown that paricalcitol produces less bone reabsorption and improves bone formation (collagen synthesis).
In 1999, Finch et al. published the first study that measured the effects of paricalcitol in bone.20 In a model using rats which had undergone parathyroidectomy who were put on a low calcium and phosphorus diet, they demonstrated that plasma calcium levels in rats indicated that paricalcitol was ten-time less potent than calcitriol in mobilizing calcium from bone (bone reabsorption). Very similar results were seen for phosphorus. They also measured urine calcium excretion and observed greater absorption in rats treated with calcitriol.
A year later, Balint et al. demonstrated in in vitro studies that although calcitriol and paricalcitol have similar effects on calcium flow from bone, at therapeutic concentrations paricalcitol did not appear to inhibit osteoblast activity.40 In 2003, Slatopolsky et al. for the first time did studies on uraemic rats and concluded that paricalcitol improved mineralization and prevented abnormal bone formation, preventing SHPT without increases in serum calcium and improving histomorphometric changes induced by uraemia and a phosphorus-rich diet (less intracortical porosity and less trabecular erosion at the level of spongy bone).12
Nakane et al., in an in vitro model, suggested that vitamin D analogues have direct effects on bone reabsorption and formation, making paricalcitol more effective than 1 alpha,25-dihydroxyvitamin D(3) and 1 alpha-hydroxyvitamin D(2) in stimulating anabolic bone formation.21 Finch et al. published the last of the studies on bone, using the same model as Slatopolsky in 2003, 5/6 nephrectomised rats who were treated with placebo, paricalcitol or cinacalcet for 6 weeks. Cinacalcet, but not paricalcitol, showed a reduction in bone volume. Cinacalcet had a similar bone formation and reduced osteoid surface but greater bone reabsorption.41 The BONAFIDE study is currently underway in CKD patients with one year of follow-up and pre- and post-cinacalcet biopsy. It is hoped that the results may help to clarify these effects.42
Other pleiotropic effects
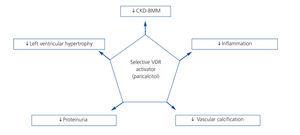
VDR receptor activation is essential for normal body function, since these receptors are present in many organs and affect several processes. This suggests a further role than just bone metabolism function (Figure 2). Given these properties, it is likely that selective VDRA-based therapy has beneficial cardiovascular effects including a reduction in the incidence of heart failure, atherosclerosis and myocardial hypertrophy, thereby decreasing morbidity and mortality.
As stated previously, differences have been found in SHPT control and biochemical parameters between selective and non-selective VDRA therapy. The differences between the therapies action on different tissues are shown below.
Calcification
The increase in vascular calcification has been associated with decreased survival in chronic haemodialysis patients. The vascular wall is composed of endothelial and vascular smooth muscle cells (VSMC) that express VDR. The presence of VDR is necessary for the health of VSMC.
In 2007, Mizobuchi et al., in an experimental severe CKD model (5/6 nephrectomy) with a phosphorus-rich diet, observed more calcification in the aorta treated with calcitriol and doxercalciferol compared to those treated with paricalcitol as well as less expression of pro-calcifying factors such as Cbfa-1 and osteocalcin after treatment for 1 month. The biochemical data on bone-mineral metabolism demonstrated that the three drugs lowered PTH. However, those treated with calcitriol showed greater serum calcium and phosphorus levels than those treated with paricalcitol. However, despite increasing phosphorus and calcium levels in the paricalcitol group, the advantageous effect of paricalcitol on other VDRA persisted.44 That same year, Cardus et al., using the same experimental model, demonstrated that the media/lumen ratio was significantly greater for the arteries of rats treated with calcitriol but was similar to that of those treated with paricalcitol compared to control rats.45 Afterwards, in 2008 Noonan et al., using the same experimental model, observed less calcification not only in the aorta, but also in the hearts of rats treated with paricalcitol (after 41 days of treatment) compared to those treated with doxercalciferol (1α-hydroxivitamin D2). In addition, the pulse wave velocity did not increase after surgery in rats treated with paricalcitol (similar values to those seen in control rats and lower than those seen in rats treated with doxercalciferol).46 Also in this same year, López et al. indicated that concomitant administration of paricalcitol and a calcimimetic in uraemic rats with SHPT had excellent control of SPTH without inducing extra-osseous calcification, preventing the mortality associated with the use of vitamin D derivatives.47 Becker et al., in a recently published study48 in which an experimental dyslipidaemia and oxidative stress model was used (apolipoprotein E KO mice), observed very similar images after 10 weeks of treatment for the two drugs with regards to calcification of the aorta and those published by Mizobuchi et al. in 2007.44
A few months ago, Guerrero et al. published the results of an in vitro study with aortic ring and in vivo in rats which received a phosphorus-rich diet who were treated with lipopolysaccharides (LPS) and calcitriol- or paricalcitol-associated LPS. This study revealed differences between both treatments with only 15 days of exposure in the rat. The treatment with paricalcitol demonstrated a greater anti-inflammatory effect than treatment with calcitriol and, unlike calcitriol, paricalcitol prevented vascular calcification.49
Very recently, the same group demonstrated a differential effect between paricalcitol and calcitriol on vascular calcification. It appears to be mediated by the distinct regulation of bone morphogenic protein and the Wnt/beta-catenin signalling pathways.50
Inflammation
It is well known that haemodialysis patients are in a chronic inflammatory state that has been associated with several complications including anaemia or malnutrition. A decrease in VDR activation is associated with elevated plasma inflammation markers: C-reactive protein (CRP) and matrix metalloproteinase.
In experimental and clinical studies, selective VDRAs have demonstrated a potential ability to modulate the inflammatory phenomenon. In a pilot study, Alborzi et al. (2008), in a randomised, double-blind, placebo-controlled study in 24 patients, observed a reduction in CRP and albuminuria in patients treated with paricalcitol via an mechanism independent of its effect on PTH.51 Recently, Navarro et al., in a study on 25 patients treated with calcitriol, found that when they were switched to paricalcitol, there was an improvement in modulation of the inflammatory state with paricalcitol with a reduction in CRP and tumour necrosis factor-alpha and an improvement in the inflammatory/anti-inflammatory cytokine ratio in patients who were previously treated with calcitriol.52 This effect, as in the Alborzi study, was independent of PTH levels.
Left ventricular hypertrophy
Left ventricular hypertrophy (LVH) in CKD is common and is associated with an increase in cardiovascular mortality in haemodialysis. Preclinical studies have revealed an association between less VDR activation and LVH.53-55
In 2007, Bodyak et al., studied cardiac function and LVH in a Dahl (salt sensitive) rat model in treatment with a sodium-rich diet and paricalcitol. They observed that the echocardiograms of rats treated with paricalcitol were similar to those of control rats. Similar findings were reported for left ventricular telediastolic pressure and cardiac renin expression.56 These same authors later re-examined retrospectively the baseline echocardiograms and those at 12 months of treatment of patients in haemodialysis who were treated or not with paricalcitol in order to corroborate the data observed in the Dahl rats. Patients who had received paricalcitol had improved diastolic function (E/A quotient) and a significant decrease in septal and posterior wall thickness. In 2007, Becker et al. reported the results of a study done in single-nephrectomy ApoE-/- rats in cardiac tissue to the American Society of Nephrology. Activation of the VDR by paricalcitol avoided the decrease in the length density of myocardial capillaries in control or untreated rats and paricalcitol also avoided the collagen expression in cardiac tissue which was seen in control rats and those treated with calcitriol.57 In 2009, Husain et al., in a severe CKD model, demonstrated that concomitant enalapril with paricalcitol reduced oxidative stress compared to Nx control rats.58 Yakupoglu et al. presented a prospective study on 76 haemodialysis patients, 36 of whom were treated with paricalcitol and 40 with calcitriol, to the European Renal Association/European Dialysis and Transplant Association congress. The patients treated with paricalcitol showed a statistically significant decrease in mean arterial pressure and LVH at the end of the study (12 months).59 A year later, Mizobuchi et al., in a uraemic rat model, demonstrated that paricalcitol reverted the LVH and fibrosis caused by the uraemia after four months of treatment, also returning VDR levels in cardiac muscle to those seen in control rats (non-nephrectomised).55 That same year, Kong et al. compared losartan and VDRA (paricalcitol and doxercalciferol) in monotherapy and in combination in a spontaneously hypertensive rat model. After two months of treatment, the combination decreased the diameter of cardiac muscle cells as well as expression of brain natriuretic peptide (BNP) and atrial natriuretic peptide.60 One year later, Bae et al., using the same experimental model as Bodyak et al., performed a comparative study between enalapril and paricalcitol in monotherapy and combination therapy. They corroborated the results for reversion of LVH, improved cardiac function (measured in this case as a variation of the shortening fraction), decreased blood BNP levels and cardiac fibrosis.61 Recently, in 2012, Thadhani et al. published the results of a placebo-controlled clinical trial in which 227 patients with CKD, mild to moderate LVH and a normal left ventricular ejection fraction and normal blood pressure who were randomised to receive paricalcitol at a starting dosage of 2µg/day or placebo for 48 weeks. No statistically significant differences were observed between the treatment groups in which LVH decreased. This was established as a primary evaluation parameter. However, they did observe a statistically significant decrease in the left atrium volume index by echocardiography, a reduction in BNP levels and a decrease in the number of hospitalisations versus the placebo arm.62
Endothelial function
In 2008, Karavalakis et al. observed a reduction in vasoconstriction in Nx 5/6 rats subjected to a special diet that induced severe hyperphosphataemia, though the histological sections of the abdominal aorta did not show positive results for vascular calcification.63 Two years later, Wu-Wong et al., in a severe CKD model by 5/6 Nx, demonstrated improvement in muscle relaxation mediated by acetylcholine after 12 days of treatment with paricalcitol.64
Renal protection and reduction in proteinuria
Pharmacological suppression of the renin-angiotensin-aldosterone system (RAAS) has been shown to reduce morbidity and mortality in patients with cardiovascular diseases and nephropathy. The most common treatment currently for proteinuria is administration of drugs that inhibit the RAAS (angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists). However, great expectation has recently been generated for the decrease in proteinuria in CKD secondary to VDR activation.
In 2005, Agarwal et al., based on the data from three randomised, double-blind, placebo-controlled studies, found a reduction in proteinuria on retrospective analysis (measured semi-quantitatively by reagent strips) in 51% of patients treated with paricalcitol versus 25% of patients treated with placebo independent of concomitant treatment with RAAS inhibitors.65 Three years later, Zhang et al. demonstrated that treatment with losartan and paricalcitol albuminuria restored the structure of the glomerular filtration barrier and markedly decreased glomerulosclerosis, thereby preventing kidney damage in a diabetic nephropathy rat model.66 In 2009, Fishbane et al., in a randomised double-blind study on 61 patients, showed that a 1µg/day dosage had a 17.6% reduction in protein excretion compared to a 2.9% increase in the control group.67 One year later, De Zeeuw et al., in a randomised, multi-site, double-blind, placebo-controlled study in 281 patients with type 2 diabetes and albuminuria, demonstrated that a starting paricalcitol dosage of 2µg/day produced an early and sustained decrease in albuminuria as measured using the albumin/creatinine ratio (approximate reduction of 20%). This effect reverted after interrupting paricalcitol treatment.68
CALCIMIMETICS
Calcimimetics are positive allosteric modulators of the calcium-sensing receptor. These drugs increase the sensitivity of the CaSR to extracellular calcium, so they reduce the levels of calcium necessary for facilitating the signalling process for this receptor, thereby causing suppression of PTH secretion.
Control of secondary hyperparathyroidism and bone mineral metabolism.
Cinacalcet is a type II calcimimetic which was the first drug in this therapeutic class indicated for the treatment of SHPT in dialysis. Phase III clinical trials carried out in haemodialysis patients revealed a decrease in serum PTH levels, a decrease in serum calcium and phosphorus levels, and a decrease in the calcium x phosphorus product in patients treated with cinacalcet. Calcium levels below 7.5mg/dl in at least two consecutive measurements were present in 5% patients, versus placebo (P<.001). These episodes were transient and rarely associated with symptoms and were controlled by modifying dosages of calcium binding agents, vitamin D analogues or both.9,69-71
In 2008, Fishbane et al. published the results of the ACHIEVE study. This was a prospective, randomised, open-label study in which 173 patients were randomly assigned to treatment with cinacalcet and low-dose vitamin D on only vitamin D analogues (paricalcitol or doxercalciferol). The study had a 6-month selection period, including a washout period, 16 weeks of dose titration and an 11-month evaluation. The percentage of patients who had a >30% reduction in PTH was greater in the group of patients that took cinacalcet than in those who were assigned to flexible vitamin D analogue dosage group (68% versus 36% P<.001). The percentage of patients who had a PTH <300pg/ml at the end of treatment in the cinacalcet group was also greater than in the vitamin D analogue group (44% versus 26%, P=.006). The percentage of subjects who achieved normal PTH ranges (150-300pg/ml) and a calcium x phosphorus product less than 55mg2/dl2 simultaneously was 21% in the cinacalcet group versus 14% in the vitamin D analogue group. This difference was not statistically significant. This was attributed to the fact that 19% of patients in the cinacalcet arm had a PTH below the normal range in the KDOQI guidelines.72
The results of the IMPACT study were published in 2012. This is a prospective, randomised, open-label study with 272 patients who underwent haemodialysis that compared the efficacy of paricalcitol in monotherapy (intravenous or oral) versus cinacalcet plus low-dose doxercalciferol/alfacalcidol (intravenous/oral). This is the first study that compared intravenous and oral administration of VDRA. The percentage of intravenous-arm patients with PTH levels between 150-300 pg/ml during week 21-28 was 57.7% in the paricalcitol group versus 32.7% in the cinacalcet group (P=.016). In the oral strata, the proportion was 54.4% in the paricalcitol group versus 43.4% in the cinacalcet group (statistically not significant difference). Four patients in the intravenous paricalcitol group (7.7%) developed hypercalcaemia and none in the oral arm (statistically not significant difference). 46.9% of patients who received intravenous cinacalcet and 54.7% of those who received this drug orally developed hypocalcaemia, the difference being statistically significant compared to the group that received paricalcitol. At the same time, a significant decrease was observed for alkaline phosphatase and bone-specific alkaline phosphatase in the paricalcitol group and an increase in the cinacalcet group. This difference was also statistically significant.73
Calcification
In 2005, Henley et al. revealed that both cinacalcet and calcitriol were effective in reducing plasma PTH levels. However, unlike calcitriol, cinacalcet did not produce hypercalcaemia, an increase in the calcium x phosphorus product or vascular calcification.74 Later, Lopez et al. observed that cinacalcet decreased elevated PTH levels in uraemic rats without inducing vascular calcification and preventing calcitriol-induced vascular calcification.75 In 2009, this same group observed that extra-osseous calcifications were partially resolved by ingesting phosphorus in an experimental trial. The use of a calcimimetic may accelerate this process by direct stimulating mineral phagocytic cells in addition to increasing urinary calcium excretion.76 The results of the ADVANCE study were recently published. This study compared the effect of administering cinacalcet plus low-dose VDRAs (selective and non-selective) versus administration of flexible dosages of vitamin D on vascular calcification and calcification of cardiac valves. No statistically significant differences were found between groups for the primary objective (percent change in the Agatston scale score for calcification of the coronary arteries). However, statistically significant differences were observed in calcification volume scores for the coronary arteries and progression of aortic valve calcification.77
Survival
Block et al. published the results of an observational study in 2010 in which rates for cardiovascular mortality or mortality for any cause in a cohort of haemodialysis patients treated with cinacalcet were lower than those observed in a cohort of patients who did not receive calcimimetics.78 The results of the EVOLVE study were recently published in which 3883 patients with moderate to severe SHPT in haemodialysis received cinacalcet or placebo in addition to conventional treatment. After 64 months in unadjusted intent-to-treat analysis, there were no differences in the primary objective of death or onset of cardiovascular events. However, a decrease was observed in parathyroidectomies.79 Unfortunately, the group randomised to cinacalcet was older (1 year older) than those who did not receive cinacalcet and a large proportion of patients who did not have to take cinacalcet ended up using it.
PHARMACO-ECONOMIC ASPECTS
The pharmaco-economic studies are contradictory. In 2004, Dobrez et al. published a retrospective study of 11443 dialysis patients that compared paricalcitol versus calcitriol in indirect terms of cost. They investigated the influence of treatment with VDRAs (paricalcitol or calcitriol) on the total number of hospital admissions, days of hospitalisation and time elapsed until the first hospitalization. The results showed a lower number of hospitalisations (-14%, P<.0001), fewer admissions per year (-0.642; P<.001) and fewer hospitalization days (-6.84; P<.001) in the group treated with paricalcitol. The cost-effectiveness analysis published by Rosery et al. in 2006 demonstrated that there was a total savings in paricalcitol treatment of 5394 euros/year, in addition to a 0.84 survival ratio at one year for patients being treated with paricalcitol and 0.80 for patients treated with calcitriol. Regarding the cost-usefulness analysis, an increase in quality of life indices was observed for treatment with paricalcitol. The authors concluded that paricalcitol was more efficient than calcitriol and alfacalcidol.80
In 2010, Shireman et al. performed a cost-effectiveness analysis with data from the ACHIEVE clinical trial mentioned previously. The dosage used in the cinacalcet plus low-dose sVDRA/VDRA arm was 49.3mg/day and 5.5µg/week, respectively, while the flexible-dose sVDRA/VDRA arm (paricalcitol/doxercalciferol) had a mean dosage of 10.5µg/week. The mean cost per patient was 5852 and 4332 dollars, respectively. Following the results of this analysis, the authors concluded that cinacalcet combined with a VDRA is not more efficient than VDRA monotherapy in achieving the primary objective of the study (percentage of patients within K/DOQI guideline range), with the cinacalcet plus low-dosages of a VDRA being more expensive.81
The pharmaco-economic analysis from the FARO study was published in 2012. These authors concluded that intravenous paricalcitol and the combination of paricalcitol plus cinacalcet showed similar effects in suppressing PTH (with no significant differences in disease severity at baseline); the costs of treatment were less in the intravenous paricalcitol group, including phosphorus chelating agents. The intravenous calcitriol group did not have any improvement in reducing PTH levels while the oral calcitriol group was effective in patients with very mild SHPT (baseline PTH 248.36pg/ml). In addition, a reduction in dosing over the courses of follow-up was only seen in the intravenous paricalcitol group.82
In the economic analysis of the IMPACT study mentioned previously, it was noted that pharmaceutical spending in the group of patients treated with paricalcitol was 41% less than in the group of patients treated with cinacalcet, including the cost of phosphorus binding agents.73
While treatment with cinacalcet was not more cost effective than the therapeutic guidelines it was compared to, there are some studies in which cinacalcet plus standard treatment was shown to be cost effective compared to standard treatment alone if the costs of dialysis are excluded.
The existence of contradictory results on the different pharmaco-economic analyses may be explained by the difference in selecting the data used in the models and by the differences between region, the use of resources and the costs reported by the respective healthcare systems.83
CONCLUSIONS
In order to approach SHPT, we have considered what we can call the patient’s “phenotype”. There are two types: VDR-activation phenotype, with normal or low serum calcium or phosphorus which allows for VDRA treatment, and the calcimimetics phenotype in which calcium and phosphorus levels are normal or elevated which has contributed to better control of SHPT in this phenotype.
There are intermediate circumstances in which the decision may tip towards one or the other side. Regarding vitamin D compounds, the use of sVDRAs such as paricalcitol appears to involve fewer episodes of hypercalcaemia, hyperphosphataemia and elevations in the calcium x phosphorus product compared to non-selective vitamin D activators (calcitriol, alfacalcidol).
The VDR activation phenotype, with SHPT in which calcium and phosphorus levels allow for the use of paricalcitol monotherapy, involves a cost efficient strategy versus the combination of this drug with cinacalcet. This drug is ideal for patients with the calcimimetic phenotype.
Activation of the VDR may have beneficial effects, not only on alterations in mineral metabolism, but also on the kidney disease itself, with the effect between sVDRAs and VDRAs being differential. Clinical and preclinical studies note that sVDRAs, and paricalcitol specifically, offer a benefit beyond controlling SHPT, demonstrating renal protection in patients with CKD and cardiovascular protection in haemodialysis compared to vitamin D.
KEY CONCEPTS
1. Activation of both the VDR and the CaSR in the parathyroid gland produces a decrease in PTH secretion and inhibition of cellular proliferation.
2. Several studies have confirmed that selective VDRA reduce PTH levels more quickly and for longer than non-selective VDRAs.
3. Selective VDRAs have less effect on intestinal calcium absorption and calcium and phosphorus mobilization in bone than non-selective VDRAs, leading to fewer episodes of hypercalcaemia and hyperphosphataemia.
4. There are studies that suggest that VDRAs are associated with greater survival in patients with CKD on haemodialysis. This benefit is greater with selective than non-selective VDRAs.26
5. Experimental studies have demonstrated that sVDRAs produce greater bone absorption and improve bone formation (collagen synthesis) compared to non-selective VDRAs.
6. The increase in vascular calcification has been associated with improved survival in chronic haemodialysis patients. Experimental studies have demonstrated that paricalcitol produces less vascular calcification than calcitriol.
7. The decrease in VDR activation is associated with an increase in plasma inflammation markers. Experimental and clinical studies have demonstrated that selective VDRAs have a potential ability to modulate the inflammatory phenomenon.
8. Preclinical studies have revealed less activation of VDR with LVH. Experimental studies in uraemic rats have shown that sVDRAs reduce LVH while the clinical results are not conclusive.
9. Several studies have demonstrated that treatment with paricalcitol is associated with a reduction in proteinuria.
Conflicts of interest
The authors attended a meeting promoted by Abbott. The objective of this meeting was scientific debate. No document was prepared for publication. Later, the authors decided to create a Working Group for the editing of this review article.
Table 1. Comparative studies of paricalcitol on bone-mineral metabolism
Figure 1. Development of secondary hyperparathyroidism in patients with chronic kidney disease (adapted from Ronco et al. 43)
Figure 2. Effects of selective activation of vitamin D receptors in chronic kidney disease (adapted from Ronco et al. 43)