Chronic musculoskeletal pain (CMP) is a very common symptom in patients with chronic kidney disease (CKD), and is associated with a significant deterioration in quality of life.
AimsTo determine the prevalence and clinical characteristics associated with CMP in patients with advanced CKD not on dialysis, and to analyse their relation with other uraemic symptoms and their prognosis significance.
Materials and methodsCross-sectional study to analyse the uraemic symptoms of an unselected cohort of patients with CKD stage 4–5 pre-dialysis. In order to characterise patients with CMP, demographic and anthropometric data were collected, as well as data on comorbidities and kidney function. In addition, inflammatory parameters, uric parameters, bone mineral metabolism including 25-hydroxycholecalciferol (25-OHCC), creatine kinase and drugs of potential interest including allopurinol, statins and erythropoiesis-stimulating agents were recorded.
ResultsThe study group consisted of 1169 patients (mean age 65±15 years, 54% male). A total of 38% of patients complained of CMP, and this symptom was more prevalent in women than in men (49 vs. 28%; P<.0001). Muscle weakness, pruritus, muscle cramps, ecchymosis, insomnia, oedema and dyspnoea were the most common symptoms associated with CMP. There were no significant associations between serum levels of creatine kinase, 25-OHCC, treatment with allopurinol, statins or erythropoiesis-stimulating agents and CMP. The female gender, elderly age, obesity, comorbidity (mainly diabetes, heart failure or COPD), and elevated levels of inflammatory markers (C-reactive protein and non-neutrophilic leukocytes) were the best determinants of CMP.
While patients with CMP showed a worse survival rate, a multivariate analysis adjusted for demographic data ruled out the independent association of CMP with mortality.
ConclusionsCMP is highly prevalent in patients with advanced CKD and is associated with other common symptoms of chronic uraemia. As with the general population, elderly age, the female gender, obesity and some comorbid conditions are the best determinants of CMP. Increased inflammatory markers commonly observed in patients with CMP may have a relevant role in its pathogenesis.
El dolor músculo-esquelético crónico (DMEC) es un síntoma muy frecuente en pacientes con enfermedad renal crónica (ERC), y contribuye de forma importante al deterioro de la calidad de vida.
ObjetivosDeterminar la prevalencia y características clínicas asociadas al DMEC en pacientes con ERC avanzada no en diálisis, analizar su relación con otros síntomas urémicos y su significado pronóstico.
Material y métodosEstudio transversal en el que se analizó la sintomatología urémica de pacientes no seleccionados remitidos por ERC estadio 4 y 5 prediálisis. Para caracterizar aquellos que presentaban DMEC, además de los datos demográficos, antropométricos, la comorbilidad y la función renal, también se recogieron parámetros de inflamación, ácido úrico, metabolismo óseo-mineral incluyendo 25-hidroxi-colecalciferol (25-OHCC), creatincinasa, y fármacos de potencial interés como alopurinol, estatinas y agentes estimulantes de eritropoyetina.
ResultadosSe incluyó a 1.169 pacientes con edad media de 65±15 años; el 54% eran hombres. Un 38% de los pacientes refería DMEC, y este síntoma fue más frecuente en mujeres que en hombres (49 vs. 28%; p<0,0001). La debilidad muscular, prurito, calambres, equimosis, insomnio, edemas y disnea fueron los síntomas más frecuentemente asociados al DMEC. No se observaron asociaciones significativas entre niveles de creatincinasa, 25-OHCC, tratamiento con alopurinol, estatinas o agentes estimulantes de eritropoyetina con DMEC. Los mejores determinantes de DMEC fueron: mujer, mayor, obesa, con comorbilidad (sobre todo diabetes, insuficiencia cardiaca o EPOC), y marcadores de inflamación elevados (proteínaC reactiva y leucocitos no neutrófilos).
Aunque los pacientes con DMEC tenían una peor supervivencia, un análisis multivariante con ajuste simple a datos demográficos descartó que el DMEC fuera un determinante independiente de la mortalidad.
ConclusionesEl DMEC es muy prevalente en pacientes con ERC avanzada, y se asocia con otros síntomas comunes de la uraemia crónica. Al igual que en la población general, características como sexo femenino, edad avanzada, obesidad y comorbilidad están más frecuentemente asociados al DMEC. La elevación de los marcadores de inflamación asociada al DMEC podría ser un hallazgo relevante para explicar su patogenia.
The symptoms of chronic kidney disease (CKD) are non-specific and very variable. Chronic musculoskeletal pain (CMP) is a very common symptom in CKD1,2 and has a significant effect on the perception of health and quality of life of patients who suffer from it.3,4
Previous studies have shown that, despite the high prevalence of CMP, it is very often undervalued and is usually attributed to different processes, related or not to chronic uraemia, such as bone and mineral disorders, neuritis, or inflammatory or degenerative osteoarthritis.4–7
The CMP in CKD is often associated with other symptoms attributable to uraemia, such as insomnia and fatigue,1–3 or to psychiatric disorders such as anxiety or depression.8 These patients need to take large doses of painkillers which, with the altered drug metabolism associated with uraemia, increases the risk of adverse reactions.9,10
Despite the importance of CMP in CKD, very few studies have analysed the clinical characteristics and determining factors. A better understanding of the origin and characteristics of the pain could help us to design more specific and effective treatment strategies.
The aims of this study were to determine the prevalence of CMP in patients with advanced CKD (ACKD) and to analyse associations with clinical and biochemical parameters, the potential relationship with drugs and the effect on survival.
Materials and methodsThis cross-sectional, observational study included incident patients referred to the ACKD clinic from January 2000 to November 2014. They were all aged over 18 and had a glomerular filtration rate of less than 30ml/min/1.73m2. No patients were excluded.
As part of the clinical interview, which was conducted by a nephrologist in all cases, information on the presence of uraemia-associated symptoms was obtained by taking a specifically focused patient history, as occurs with all patients in our ACKD clinic according to protocol. The symptoms included were: anorexia; nausea or vomiting; oedema; dyspnoea; decreased physical exercise; fatigue (muscle weakness); pruritus; ecchymosis or epistaxis; cramps; cold intolerance; night-time insomnia; daytime drowsiness; restless legs; myoclonus; and CMP.
CMP was defined as the presence of muscle or bone pain in any location (upper or lower limbs or trunk) for more than three months, not attributable to trauma, and requiring analgesic therapy at least three times a week. The intensity of the CMP was not collected as data for the study.
Cramp was defined as involuntary, sustained and painful contraction of muscles or muscle groups in the lower or upper limbs, which occurred spontaneously and essentially at rest.
To characterise patients with or without pain, in addition to demographic data and body mass index, the following were included as variables of potential interest: the Davies comorbidity index; haemoglobin; total leucocyte and neutrophil counts; MDRD estimated glomerular filtration rate (eGFR); and plasma concentrations of uric acid, calcium, phosphorus, bicarbonate, PTH, alkaline phosphatase, serum albumin and C-reactive protein. All the clinical biochemistry parameters were determined using conventional laboratory methods.
Previous history of gouty arthritis and the use of drugs of interest for their potential relationship with CMP, such as statins, allopurinol and erythropoiesis stimulating agents (ESA), were also included.
In a subgroup of 671 unselected patients, serum creatine kinase (CK) was determined to study the association between rhabdomyolysis and CMP or cramps.
In another subgroup of 361 unselected patients, serum levels of 25-hydroxy-cholecalciferol were determined for comparison between patients with and without CMP.
Study design and statistical analysisIn this cross-sectional study, we describe the prevalence of CMP, association with other symptoms, clinical and biochemical characteristics of patients who suffer from CMP, and the most significant differences compared to the rest of the study group. In addition, to establish whether CMP is predictive of patient outcome, a univariate comparison was made of survival of patients with and without CMP, adjusted for age and gender.
Descriptive statistics are presented as mean and standard deviation or as median and interquartile ranges (IQ) for continuous variables and as percentages for categorical variables.
For comparison of continuous variables, parametric tests (Student's t-test) or non-parametric tests (Mann–Whitney) were used, depending on the type of distribution, while the chi-square test was used for categorical variables.
To establish the independent association of the study variables with CMP in the study group, multivariate logistic regression was used. A first analysis was performed to determine the symptoms best associated with CMP. The following independent variables were included in another separate model: age; gender; comorbidity index; diabetes mellitus; body mass index; leucocytes; neutrophils; uric acid; calcium; phosphorus; bicarbonate; PTH: C-reactive protein; and treatment with statins, allopurinol or EPO. Automated stepwise regression was used for the selection of variables with the best predictive models.
To analyse the differences in survival, Kaplan–Meier curves (univariate analysis) and a Cox multivariate proportional hazard model were used to adjust for differences in survival with age and gender only (a more exhaustive adjustment was not justified).
A p<0.05 was considered as statistically significant, and all the p values shown are bilateral. Statistical analyses were performed with the IBM-SPSS 21.0 program (IBM Corp. Armonk, USA).
ResultsPrevalence of chronic musculoskeletal pain and associated symptomsThe demographic, clinical and clinical biochemistry characteristics of the 1169 patients included in the study are shown in Table 1.
Patient characteristics for the whole group and the subgroups with or without chronic musculoskeletal pain.
| Total | No pain | Pain | p | |
|---|---|---|---|---|
| No. of patients | 1169 | 728 (62%) | 441 (38%) | |
| Age (years) | 65 (15) | 62 (16) | 70 (12) | <0.000 |
| Gender (% male) | 54 | 63 | 41 | <0.000 |
| Comorbidity index: none; mild/moderate; severe (%) | 32/52/16 | 37/49/14 | 24/56/20 | <0.000 |
| Diabetes mellitus (%) | 37 | 34 | 41 | 0.012 |
| BMI (kg/m2) | 29.3 (5.8) | 28.4 (5.4) | 30.8 (6.1) | <0.000 |
| Haemoglobin (g/dl) | 11.5 (3.9) | 11.6 (4.7) | 11.3 (1.6) | 0.132 |
| Total leucocytes (mm3) | 8048 (2.63) | 7925 (2.64) | 8250 (2.62) | 0.041 |
| Neutrophils (mm3) | 5411 (2.20) | 5381 (2.30) | 5459 (2.03) | 0.556 |
| eGFR, ml/min/1.73 m2 | 14.4 (4.9) | 14.4 (4.9) | 14.5 (5.0) | 0.630 |
| Uric acid (mg/dl) | 7.6 (1.9) | 7.7 (1.9) | 7.4 (1.9) | 0.035 |
| Total calcium (mg/dl) | 9.13 (0.79) | 9.11 (0.83) | 9.18 (0.74) | 0.139 |
| Phosphorus (mg/dl) | 4.73 (1.11) | 4.77 (1.18) | 4.67 (0.99) | 0.130 |
| Bicarbonate (mmol/l) | 21.6 (4.0) | 21.4 (4.2) | 21.7 (3.6) | 0.252 |
| PTH (pg/ml) | 260 (219) | 265 (222) | 252 (214) | 0.307 |
| PTH<100pg/ml (%) | 22 | 21 | 22 | 0.820 |
| PTH>500pg/ml (%) | 11 | 12 | 10 | 0.369 |
| Alkaline phosphatase (IU/ml) | 105 (59) | 102 (56) | 108 (63) | 0.090 |
| Serum albumin (g/dl) | 3.87 (0.54) | 3.88 (0.57) | 3.86 (0.49) | 0.456 |
| C-reactive proteina (mg/l) | 3.85 [13–11.04] | 3.52 [1.27–9.35] | 4.45 [1.72–13.31] | 0.001 |
| C-reactive protein >5mg/l (%) | 43 | 39 | 48 | 0.003 |
| History of gouty arthritis, (%) | 10 | 10 | 10 | 0.898 |
| Statins (%) | 53 | 52 | 54 | 0.357 |
| Allopurinol (%) | 23 | 22 | 25 | 0.281 |
| ESA-EPO (%) | 62 | 62 | 61 | 0.688 |
Of the patients studied, 441 (38%) had CMP. CMP was more common among females than males (49% vs 28%; p<0.0001).
The majority of the patients used paracetamol or metamizole for analgesia. At the time of their first consultation, over 20% of the patients with CMP were also assiduously taking nonsteroidal anti-inflammatory drugs. Less than 15% were mixing paracetamol with tramadol or codeine. Less than 5% of the patients with CMP were being treated with opioids (fentanyl or other similar drugs) or pain-perception modifiers (gabapentin or pregabalin); the significant adverse effects suffered by the patients treated with these drugs are worth mentioning.
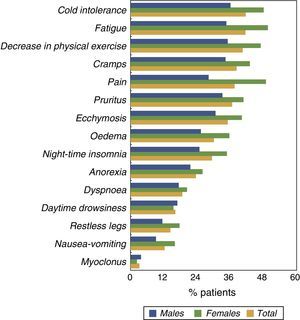
The prevalence of other symptoms is shown in Fig. 1 according to gender. The majority of the symptoms were more prevalent among females than males. Apart from CMP, other symptoms that were notable due to their high prevalence were fatigue, poor physical activity level, cold intolerance and pruritus.
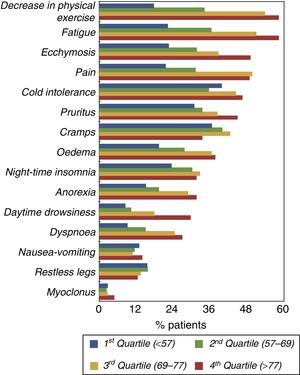
The prevalence of other symptoms is shown in Fig. 2 according to age quartile. There was a marked increase in CMP and other symptoms like fatigue and decreased physical exercise in the top 2 age quartiles (aged>69).
By logistic regression, the symptoms most often associated with CMP were (Table 2) fatigue, pruritus, cramps, ecchymosis, insomnia, oedema and dyspnoea.
Multivariate logistic regression. Uraemic symptoms most associated with the chronic musculoskeletal pain.
| Variable | Odds ratio | 95% CI OR | p |
|---|---|---|---|
| Fatigue (muscle weakness) | 2.91 | 2.22–3.81 | <0.000 |
| Pruritus | 1.75 | 1.34–2.28 | <0.000 |
| Cramps | 1.62 | 1.24–2.11 | <0.000 |
| Oedema | 1.59 | 1.19–2.11 | 0.001 |
| Ecchymosis | 1.55 | 1.18–2.03 | 0.002 |
| Night-time insomnia | 1.38 | 1.04–1.84 | 0.027 |
| Dyspnoea | 1.41 | 1.01–1.97 | 0.049 |
The following did not fit into the best model: anorexia; nausea/vomiting; poor physical activity level; cold intolerance; myoclonus; restless legs; daytime drowsiness.
In order to investigate whether or not rhabdomyolysis could cause or contribute to CMP, we included the CK plasma concentrations from analyses of 671 non-selected patients (CK started to be measured in all patients from 2006 onwards).
No significant differences were found between patients with or without CMP in mean CK concentration according to gender (males with or without CMP: 124±120 vs 140±169U/ml; females with or without CMP: 91±59 vs 94±79U/ml).
Nor was there a higher rate of CK above normal limits (>194U/ml) in patients with CMP versus those without CMP (9.7 vs 14.4%).
We did find a higher mean plasma concentration of CK in men who had cramp compared to those not suffering from cramp (161±163 vs 120±149U/ml; p=0.012). In women with cramp, CK levels were also higher, although compared to women without cramp, the difference was only bordering on statistical significance (101±72 vs. 86±67U/ml; p=0.058).
CK elevation above normal was more common in all patients (male and female) with cramp than those who did not suffer from cramp (17.4% vs 9.3%; p=0.002).
In 391 patients treated with statins and in whom CK was determined, CK levels were significantly higher than in the 280 patients not taking statins (133±135 vs 94±111U/ml; p<0.0001). Also, a CK concentration above the normal limit was more common among patients on statins than among those not on statins (17 vs 6%; p<0.0001).
Serum levels of 25-hydroxycholecalciferol and chronic musculoskeletal painNo significant differences were found in 25-hydroxycholecalciferol levels determined in 203 patients without CMP and 158 patients with CMP: 14.9±8.4 vs 13.4±8.5ng/ml; p=0.08.
Nor were significant differences found between those with or without CMP in the percentage of patients with serum 25-hydroxycholecalciferol levels below 20ng/ml (82 vs 77%; p=0.317).
Determining factors in chronic musculoskeletal painTable 1 shows the demographic, clinical and clinical biochemistry characteristics of the patients with or without CMP.
CMP was most common in older women with comorbidity. In addition to diabetes mellitus, other conditions significantly associated with CMP were heart failure (100% of the patients with heart failure had CMP) and COPD (46% had CMP). In contrast, other comorbidities, such as coronary heart disease, CVA or peripheral ischaemia, or a history of gouty arthritis were not associated with higher rates of CMP.
Patients with CMP were more obese, had higher C-reactive protein and total leucocyte levels than the other patients, but no differences were found in clinical biochemistry parameters that characterise metabolic bone disease.
No differences were found in the prescribing of statins, allopurinol or EPO.
By multivariate logistic regression, the best predictive model for CMP (Table 3) had the following characteristics: female; elderly; obese; comorbidity; and elevated inflammatory markers (C-reactive protein and non-neutrophil leucocytes).
Multivariate logistic regression. Clinical and analytical characteristics best associated with the chronic musculoskeletal pain.
| Variable | Odds ratio | 95% CI OR | p |
|---|---|---|---|
| Male gender | 0.458 | 0.350–0.600 | <0.000 |
| Age (×10 years) | 1.31 | 1.20–1.42 | <0.000 |
| Comorbidity index 0=none, 1=mild/moderate, 2=severe | 1.282 | 1.038–1.584 | 0.021 |
| BMI (×10kg/m2) | 1.55 | 1.31–1.79 | 0.000 |
| Leucocytes (×1000/mm3) | 1.303 | 1.136–1.494 | <0.000 |
| Neutrophils (×1000/mm3) | 0.733 | 0.620–0.865 | <0.000 |
| C-reactive protein (×10mg/l) | 1.09 | 1.01–1.16 | 0.028 |
The following did not fit into the best prediction model: uric acid; bicarbonate; albumin; PTH; alkaline phosphatase; diabetes; statins; ESA-EPO; allopurinol; and glomerular filtration rate.
We followed up the progress of 1078 patients, collecting data on death from any cause and date with censoring for end of follow-up (February 2014), loss to follow-up or kidney transplantation.
The median follow-up time was 1075 days (IQ ranges: 456–1756 days). During this period, 489 patients died (42% of the study population).
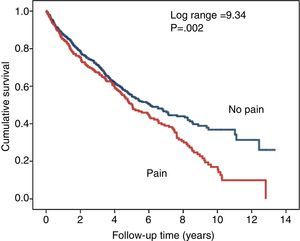
Fig. 3 shows the survival curves for the patients who had CMP as an initial symptom and those who did not. Although the difference proved to be significant in the univariate comparison, when just age and gender were included in a Cox proportional hazard regression model, the association between CMP and mortality was no longer significant (Wald 0.193; p=0.661).
DiscussionThis study shows CMP to be a highly prevalent symptom in patients with CKD, which is more common in older, obese women with comorbidity, especially diabetes, heart failure and COPD, and associated with inflammatory markers. Patients with CMP were also more likely to have fatigue, pruritus, cramps, insomnia, oedema and dyspnoea. In contrast, CMP did not appear to be associated with alterations in bone and mineral metabolism, or significant differences in plasma levels of vitamin D, rhabdomyolysis, use of drugs (statins, EPO) or gouty arthritis.
It is estimated that 10–20% of the general adult population suffers from some form of chronic pain.11–13 It is reported to be more common among older women, with the prevalence heavily influenced by physical factors (obesity, comorbidity), emotional factors (separation, divorce, widowhood), psychological factors (anxiety, depression) and social factors (education, employment and income).14 Around 50–70% of these pain syndromes are of musculoskeletal origin (CMP), signifying that somewhere in the range of 5–14% of the general population is affected by CMP.11–14 In fact, one study in Spain states that over 20% of the general population suffers from CMP.15
Despite the already high prevalence in the general population, the figures observed in our population with CKD were much higher still, making it extremely unlikely that this finding could be attributable to a variation within normal limits.
It has been reported that CMP is very prevalent in patients on dialysis,1,3,4 but also in CKD in predialysis stages,2,5,16 and even in kidney transplant patients.10
As with the general population,17,18 in this study, we also found a higher prevalence of CMP among women than among men. The reason for CMP being more likely to occur in females is not fully understood. Apart from the fact that women tend to be much more explicit than men when giving their medical history (subjective assessment of the authors), which might also explain the higher prevalence of other symptoms in the women compared to the men in our study population, there is a hypothesis that females have greater sensitivity to pain through mechanisms that may be related to both peripheral and central perception systems.18
In this study, we ruled out CMP being associated with rhabdomyolysis, a disease that was diagnosed from serum CK levels. However, we did find abnormally high CK levels in patients treated with statins and in those who reported frequent cramps.
Administration of EPO has been found to cause flu-like illness with increased sensitivity to pain.19 In this study, we did not find a higher rate of treatment with ESA-EPO in patients with CMP.
In a study conducted in Asian patients with CKD, hyperuricaemia and gouty arthritis were found to be associated with an increased incidence of CMP.5 In our study, we observed no significant associations between CMP and uric acid concentrations in blood, treatment with xanthine-oxidase inhibitors, or history of gouty arthritis.
An association has been reported between reduced levels of blood vitamin D (25-hydroxycholecalciferol) and CMP in the general population.20 Low serum levels of vitamin D are very common in CKD and, although the patients with CMP in our study had lower levels, the difference with respect to those without CMP was not statistically significant.
The prevalence of CMP increased in patients with comorbidity; particularly marked was that associated with heart failure (100% of the cases). This finding also helps explain the association found between CMP and both oedema and dyspnoea. Diabetes mellitus and COPD are also associated with CMP. This increased prevalence of pain has previously been reported in the literature in COPD patients21
One surprising finding in this study was the lack of association between bone-mineral biochemical alterations and CMP. Other studies have found a relationship between CMP and adynamic bone disease,22 and also with high-remodelling bone disease6,7,22; the latter of the two also being characterised by the fact that the pain tends to be more intense.
In this study, CMP was associated with poorer survival compared to those who did not have CMP. The association between CMP and mortality rates in CKD has also been demonstrated in another study.23 However, when we adjusted the proportional hazard model simply with gender and age in our patients, the statistical significance of CMP on the prediction of survival was cancelled out.
As occurs in the general population,24 the CMP in our patients was associated with higher levels of inflammatory markers, and this finding could help us to understand the possible mechanisms underlying the development of pain in CKD.25–27
Chronic inflammatory states are capable of sensitising peripheral nociceptors (pain receptors) due to the numerous inflammatory mediator substances (interleukins, TNF, prostaglandins, etc.).27 The sensitisation of nociceptors can sometimes be so intense that they can end up being activated spontaneously without the mediation of any injury.27
These same inflammatory mediators, and perhaps certain uraemic toxins, may also play an important role in the sensitisation of pain perception in the central nervous system and the balance of endogenous pain inhibitors (endorphins, endocannabinoids, GABA, etc.).25–27
This study has certain limitations. It is a cross-sectional study, in which we did not estimate the pain intensity, and nor did we assess or describe the severity of musculoskeletal damage and its relationship with the prevalence of CMP. Moreover, we did not collect information on the patients’ mental health status or on their social/economic/cultural circumstances, and that could affect the interpretation of the results.
In conclusion, CMP is highly prevalent in patients with advanced CKD and is associated with other symptoms common in chronic uraemia. The main characteristics of the patient with CMP are: older female; obesity; certain comorbidities; and elevated inflammatory markers.
Further studies are needed to analyse what type of analgesics or what additional supportive measures could be more effective and less toxic in this population.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Caravaca F, Gonzales B, Bayo MA, Luna E. Dolor músculo-esquelético en pacientes con enfermedad renal crónica. Nefrologia. 2016;36:433–440.