Peritoneal infection is a common problem that has a negative impact on the survival of patients and the technique. The early administration of peritoneal infection treatment reduces complications.
The goal of this study is to propose a multicomponent index (MUL+DO) for the quick and efficient diagnosis of peritoneal infection.
We selected a training cohort of peritoneal effluent samples which were analyzed by Multistix® 10 SG Siemens test strips for leukocyte detection. Then, each sample was examined according to the gold standard: number of leukocytes, polymorphonuclear percentage and microbiological culture. We constructed the MUL+DO index by adding one point to the MULTISTIX [0-1-2-3] modified chromatic scale if the patient reported pain. The MUL+DO index ranged from 0 to 4. A model validation cohort was then created. MUL+DO was applied to each sample and leukocytes and polymorphonuclear percentage were also assessed.
The training cohort ultimately included 134 samples, 34 of which with infection (25.4% [17.6–33.1]). Samples with a MUL+DO value greater than 1 presented a sensitivity and specificity of 100%. The validation cohort included 100 samples with 16 infections (16% [8.3–23.7]).
Assuming a sample with a MUL+DO value greater than 1 to be positive, we obtained a sensitivity of 100% and a specificity of 95.2%.
The MUL+DO index applied to the training cohort showed a perfect separation of the positive and negative populations. All positive patients presented a score ≥2. In the validation cohort, the MUL+DO reported a sensitivity of 100% and a specificity of 95.2%.
La infección peritoneal es un problema frecuente que afecta negativamente a la supervivencia del paciente y de la técnica. El inicio rápido del tratamiento de la infección peritoneal reduce las complicaciones.
Se busca diseñar un índice multicomponente (MUL+DO) para el diagnóstico rápido y eficiente de infección peritoneal.
Con ese objetivo, se crea una cohorte de construcción con muestras de efluente peritoneal que se analizaron con tiras Multistix ® 10 SG Siemens para la detección de leucocitos. Después, se examinó cada muestra según el patrón oro: número de leucocitos, porcentaje de polimorfonucleares y cultivo microbiológico. Se construyó MUL+DO sumándole un punto a la escala cromática modificada MULTISTIX [0-1-2-3] si el paciente reporta dolor. MUL+DO toma valores de 0 a 4. Posteriormente, se creó una cohorte de validación del modelo. MUL+DO se aplicó a cada muestra, determinándose también leucocitos y porcentaje de polimorfonucleares.
La cohorte de construcción incluyó 134 muestras, 34 tenían infección (25,4% [17,6–33,1]). Las muestras con un valor MUL+DO >1, presentaron una sensibilidad y especificidad del 100%. La cohorte de validación incluyó 100 muestras con 16 infecciones (16% [8,3-23,7]).
En la cohorte de validación asumiendo como positiva una muestra con un valor MUL+DO > 1, se obtuvo una sensibilidad del 100% y una especificidad del 95,2%.
MUL+DO aplicado en la cohorte de construcción, mostró una separación perfecta de las poblaciones positiva y negativa. Todos los pacientes positivos presentaron una puntuación ≥2. En la cohorte de validación, el índice MUL+DO presentó una sensibilidad del 100% y una especificidad del 95,2%.
The impact of peritonitis (IP) within a peritoneal dialysis (PD) unit is determined by the effect on the patient's long-term survival (not by its direct effect on lethality rate1), the close relationship with the failure of the technique,2 and the loss of residual renal function.3
Although the incidence of IP in PD has decreased in recent years, possibly due to advances in systems of tubing connection, control of carriers of nasal Staphylococcus aureus,4,5 improvement of modalities,6 use of more biocompatible solutions7–9 and innovations in the care of the exit orifice,10 the peritoneal infection, still remains the most frequent complication in PD.
The diagnosis of “typical” bacterial peritonitis is based on the peritoneal liquid turbid effluent, specifically in a leukocyte (L) cell count greater than 100L/microliter (L/μL) and more than 50% polymorphonuclear (PMN) in the formula.11
In the case of “atypical” IP, the presence of at least two of the following three conditions is required12:
- •
Abdominal symptoms, mainly pain.
- •
Cloudy effluent liquid, with the cell count and formula specified above.
- •
Gram stain or peritoneal fluid culture demonstrating the presence of microorganisms.
A rapid onset of antibiotic treatment, even without waiting for confirmation of diagnosis, is essential to reduce the complications of IP.11
The test strips, commonly used for the diagnosis of urinary infection, have shown excellent diagnostic validity (in terms of sensitivity and specificity) for the diagnosis of IP in PD,13–17 although without reaching 100% sensitivity and specificity.
The frequent multicomponent nature of the diagnostic tests18 (in the sense that, usually, more than one characteristic must be attended) led us to consider the construction of a multicomponent model (MUL+DO) for the diagnosis of peritoneal infection.
The MUL+DO model is formed, by the use of Multistix® 10 SG Siemens test strips, for the detection of leukocytes in effluent peritoneal fluid and self-reported pain by the patient. With this objective, a cohort was used to build the model (CCM), with subsequent validation in another independent cohort (CVM). The resulting model should be useful in clinical practice if it is quick, simple and valid.
MethodsTo construct the MUL+DO model, between January 1 and June 30, 2014, information was collected on all peritoneal effluent samples obtained after minimum of a 2-h of peritoneal fluid in the cavity and a minimum infusion volume of 1500cc.
The samples were analyzed using Siemens Multistix® 10 SG test strips, the square containing the leukocyte esterase reagent was instilled or immersed in peritoneal fluid. The results were recorded according to the modified chromatic scale that goes from the white to the most intense violet (value 0=0–15L/L, value 1=16–70L/μL, value 2=71–125μL/L value 3=126–500L/μL). Other collected variables were: age, sex, number of previous peritonitis, turbid fluid (no/yes/doubtful), pain (self-reported do you have abdominal pain? Yes/no) and diabetic condition (yes/no).
Subsequently, each sample was evaluated according to the gold standard, cell count of L greater than 100L/μL and a formula with more than 50% of PMN.11 Cytology analysis of the peritoneal effluent was performed: the cell count of leukocytes and red blood cells was obtained, in improved Neubauer chamber, the differential count (formula) was carried out by extension and staining with May–Grünwald–Giemsa. A microbiological culture of each sample was also performed. Finally, the concordance between the “gold standard” and the first analysis based on the test strips was analyzed.
The construction of the MUL+DO multicomponent model was based on the high association between the Multistix test strips and the IP. To the values obtained in the modified chromatic scale (MULtistix [0-1-2-3]), one point was added if the patient reported pain (Dolor +1). Finally, the MUL+DO scale presents values from 0 (less risk) to 4 (more risk).
To validate the MUL+DO model, from July 1st to December 31st 2014, samples were collected following the same procedure and the information obtained from all patients was identical. Subsequently, each sample was evaluated according to the gold standard of “typical” peritonitis (number of leukocytes and percentage of PMN) and also performing a microbiological culture.
Samples collected for either CCM or CVM were excluded if the patient had antibiotic treatment during the previous month.
This study protocol and the written informed consent for each patient were approved by the Ethics Committee of the Central University Hospital of Asturias.
Statistic analysisAge is shown as mean±standard deviation. The equality between means was compared by the Student–Welch test. The rest of the variables, that were all categorical, were described by absolute and relative frequencies, reporting a confidence interval of 95% for the incidence of infection. The comparison of them was made by the chi square exact test. Sensitivity and specificity for Multistix and for the final score are reported. Diagnostic capacity was measured by the area under the curve Receiver Operating Characteristics (ROC). p-Values below 0.05 were considered statistically significant. The statistical analyses were carried out with the free distributed software R (www.r-project.org).
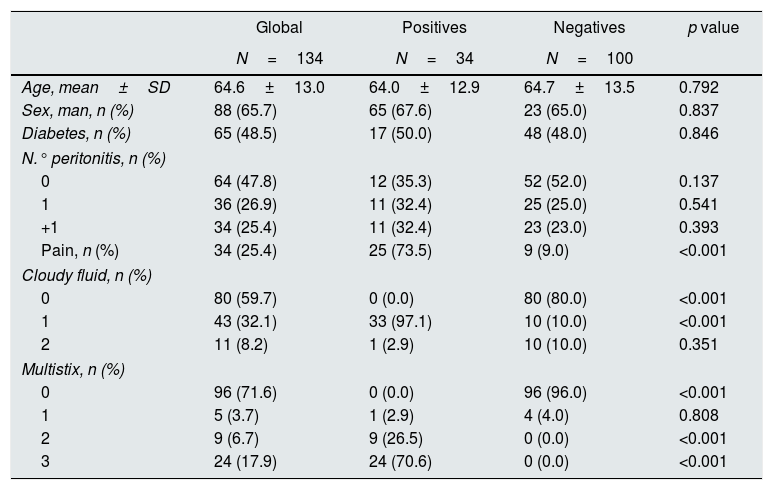
ResultsThe CCM included 134 samples, 34 of them presented infection (25.4% [17.6–33.1]). Table 1 shows the description of the populations as positive (with infection) and negative (without infection); the average age was 64.6±13.0, 66% being men. The positive samples presented more pain, that was significant, more cloudy liquid and more coloration in the Multistix test strips.
Description of the variables considered in the model construction cohort (CCM). Standard deviation (SD). Number of previous peritonitis (N. peritonitis). Turbid liquid no (0) yes (1) doubtful (2).
| Global | Positives | Negatives | p value | |
|---|---|---|---|---|
| N=134 | N=34 | N=100 | ||
| Age, mean±SD | 64.6±13.0 | 64.0±12.9 | 64.7±13.5 | 0.792 |
| Sex, man, n (%) | 88 (65.7) | 65 (67.6) | 23 (65.0) | 0.837 |
| Diabetes, n (%) | 65 (48.5) | 17 (50.0) | 48 (48.0) | 0.846 |
| N.° peritonitis, n (%) | ||||
| 0 | 64 (47.8) | 12 (35.3) | 52 (52.0) | 0.137 |
| 1 | 36 (26.9) | 11 (32.4) | 25 (25.0) | 0.541 |
| +1 | 34 (25.4) | 11 (32.4) | 23 (23.0) | 0.393 |
| Pain, n (%) | 34 (25.4) | 25 (73.5) | 9 (9.0) | <0.001 |
| Cloudy fluid, n (%) | ||||
| 0 | 80 (59.7) | 0 (0.0) | 80 (80.0) | <0.001 |
| 1 | 43 (32.1) | 33 (97.1) | 10 (10.0) | <0.001 |
| 2 | 11 (8.2) | 1 (2.9) | 10 (10.0) | 0.351 |
| Multistix, n (%) | ||||
| 0 | 96 (71.6) | 0 (0.0) | 96 (96.0) | <0.001 |
| 1 | 5 (3.7) | 1 (2.9) | 4 (4.0) | 0.808 |
| 2 | 9 (6.7) | 9 (26.5) | 0 (0.0) | <0.001 |
| 3 | 24 (17.9) | 24 (70.6) | 0 (0.0) | <0.001 |
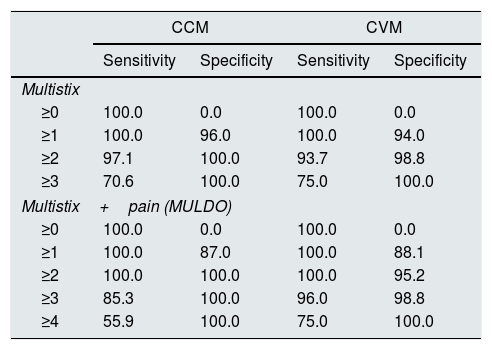
Table 2 (left) presents the results of sensitivity and specificity of Multistix (0-3) and of the model called MUL+DO (in which to the previous score one point is added if the patient reports pain, so the final score is 0–4). Assuming as positive (IP) a sample with a MUL+DO value greater than 1, the sensitivity and specificity is 100%.
Sensitivity and specificity of the two cohorts. Model construction cohort (CCM). Model validation cohort (CVM).
| CCM | CVM | |||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| Multistix | ||||
| ≥0 | 100.0 | 0.0 | 100.0 | 0.0 |
| ≥1 | 100.0 | 96.0 | 100.0 | 94.0 |
| ≥2 | 97.1 | 100.0 | 93.7 | 98.8 |
| ≥3 | 70.6 | 100.0 | 75.0 | 100.0 |
| Multistix+pain (MULDO) | ||||
| ≥0 | 100.0 | 0.0 | 100.0 | 0.0 |
| ≥1 | 100.0 | 87.0 | 100.0 | 88.1 |
| ≥2 | 100.0 | 100.0 | 100.0 | 95.2 |
| ≥3 | 85.3 | 100.0 | 96.0 | 98.8 |
| ≥4 | 55.9 | 100.0 | 75.0 | 100.0 |
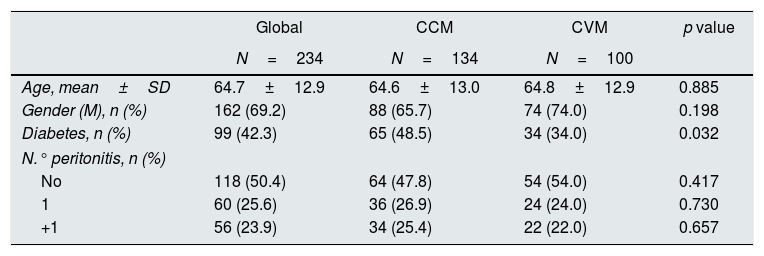
The CVM included 100 samples with 16 infections (16% [8.3–23.7]), the difference in incidence observed in both cohorts was not statistically significant (p=0.107). Both cohorts were similar, with a lower presence of diabetes in the validation cohort (Table 3). Table 2 (right) presents the sensitivity and specificity observed in the validation cohort. Assuming as positive a sample with a value of MUL+DO greater than 1, the sensitivity was 100% and the specificity 95.2%.
Variables studied in the two populations. Gender male (gender [M]).
| Global | CCM | CVM | p value | |
|---|---|---|---|---|
| N=234 | N=134 | N=100 | ||
| Age, mean±SD | 64.7±12.9 | 64.6±13.0 | 64.8±12.9 | 0.885 |
| Gender (M), n (%) | 162 (69.2) | 88 (65.7) | 74 (74.0) | 0.198 |
| Diabetes, n (%) | 99 (42.3) | 65 (48.5) | 34 (34.0) | 0.032 |
| N.° peritonitis, n (%) | ||||
| No | 118 (50.4) | 64 (47.8) | 54 (54.0) | 0.417 |
| 1 | 60 (25.6) | 36 (26.9) | 24 (24.0) | 0.730 |
| +1 | 56 (23.9) | 34 (25.4) | 22 (22.0) | 0.657 |
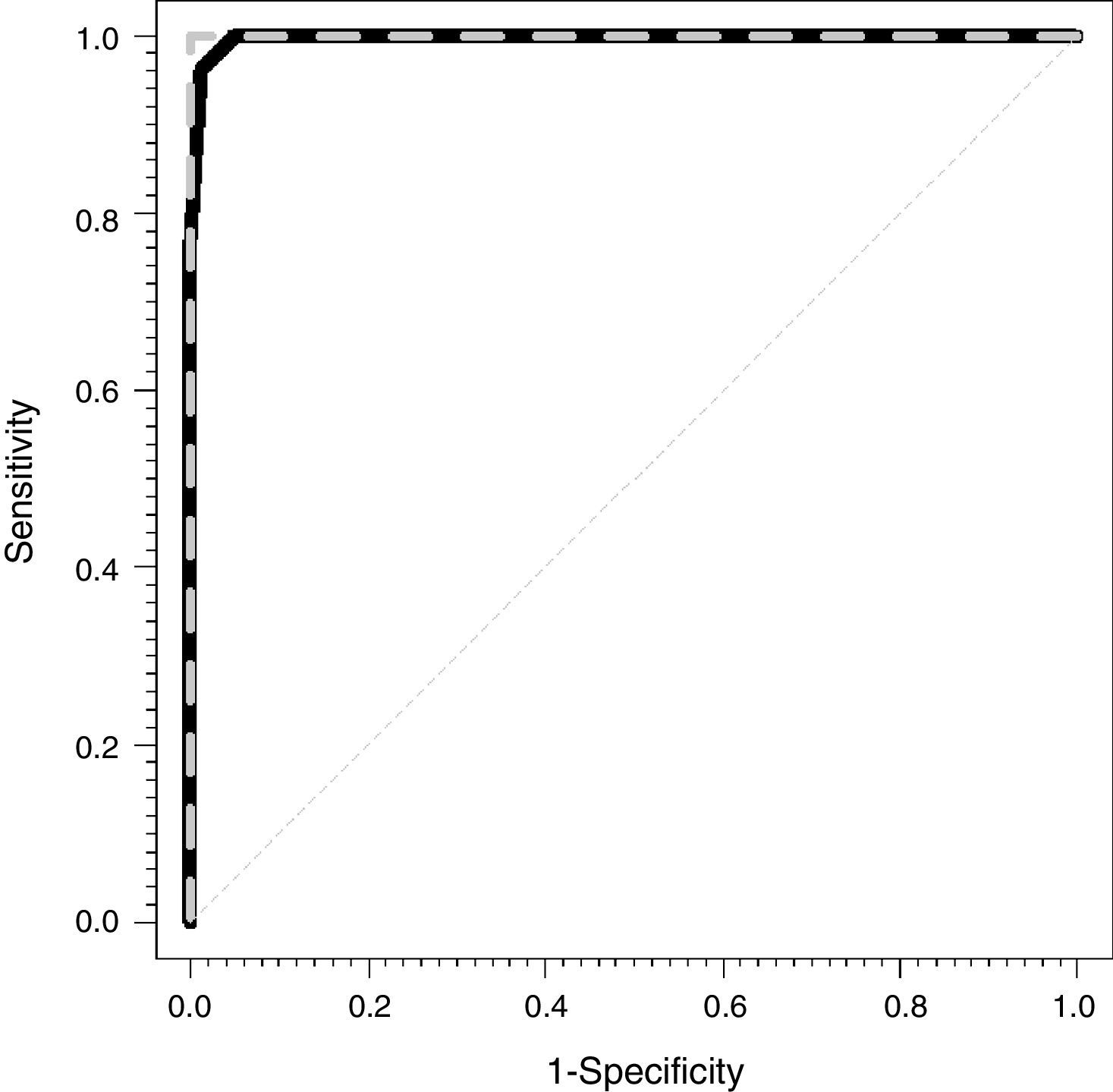
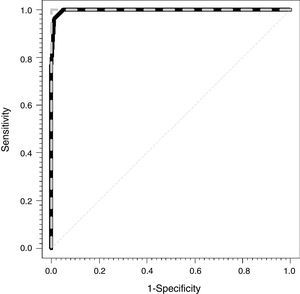
Fig. 1 shows the ROC curves for the CCM (dashed gray line) and for the CVM (solid black line). The areas under the observed curve were 1 and 0.997 (0.991–1) for the CEM and the CVM, respectively.
DiscussionThe application of the multicomponent model MUL+DO (Multistix [0-1-2-3]+pain [0–1]) in patients from the construction cohort, showed exact separation between the population with positive IP and negative IP. All positives have a score equal to or greater than two, while all negatives had a score equal to or less than one. The inclusion of the variable pain, easy and fast to collect and present in 25 of 34 positive and only in 9 of 100 negative, allows to diagnose with greater reliability, the presence of peritonitis.
Other weights of the variables included could have been considered, however, given the high quality of the proposed method and the simplicity of its calculation, the final choice was not to include different weights.
In the validation cohort, the MUL+DO model presented very good results, although not totally exact as in the previous case. All positive samples had a value greater than or equal to two (sensitivity of 100%), although of the 84 negative individuals included in this cohort, three had a score of two and one had a score of three, resulting in a specificity of 95.2%. This is an acceptable error, taking into account that in daily clinical practice the exact diagnostic test does not exist.18,19
The cut-off point was established at a value greater than or equal to two, based on the ROC20 curve and according to Galem and Gambino21 postulates: “The point of greatest sensitivity is chosen when dealing with treatable serious diseases and the results false positives do not involve psychological or economic trauma for the individuals examined.”
The use of a multi-component predictive model type MUL+DO in the diagnosis of spontaneous bacterial peritonitis, in the field of hepatology, could be the subject of research, although the articles consulted show that the validity of the model is controversial (sensitivity and specificity22–25), if the diagnosis of spontaneous bacterial peritonitis is based only in test strips.
Despite the good results obtained, caution should be exercised regarding the use of MUL+DO by the patient. Poor preservation of the Multistix® 10 SG Siemens strips, misinterpretation of the results (patients with poor vision, on the strip other determinations appear: glucose, blood, etc.), etc. may reduce the reliability of the test,26 an consequently its validity and clinical performance.
In clinical practice, the diagnosis of peritonitis is difficult without knowing the leukocyte formula in cases with “atypical” formulas where lymphocyte or eosinophilic predominance exists. MUL+DO is a diagnostic test used to determine, in two min, whether the patient has peritoneal infection or not. This, should not preclude, that samples of peritoneal effluent are sent, for counting and leukocyte formula, as well as for microbiological culture, which will help, later on, to know the type of IP.11,12
The differences between the two cohorts CCM and CVM do not imply the existence of any bias. The lower incidence of PI in the CVM could be justified by the lower presence of diabetics27,28 or the possible seasonal variability of peritonitis.29,30
Although it is not the objective of this study, we would like to show inherent characteristics of the test, which communicate its feasibility of application.18 Its simplicity facilitates its acceptability by both patient and the health personnel, it can be used by personnel trained in its management (caregiver, patient), not necessarily health personnel. The cost of each strip Multistix 10 SG®, is approximately 0.3 euros. It does not need infrastructure (laboratory), it can be done at the bedside. The results are obtained in 2min, allowing the early initiation of treatment once the diagnosis is known, together with its validity (sensitivity 100% and specificity 95.2).
Taking into account that both the CCM and the CVM were collected in the same center, an external validation would provide more scientific evidence.
Finally, we conclude that the MUL+DO index is a (a) rapid test (2min), (b) low cost (30euro cents), (c) and simple (does not require infrastructure). The CCM, presented an absolute diagnostic validity, both the negative and positive samples were exactly classified (specificity and sensitivity 100%). Although, the validation of the model presented slightly worsened the results (sensitivity 100% and specificity 95.2%).
Conflict of interestThe authors declare that they have no conflicts of interest.
To the laboratory of the Central University Hospital of Asturias for their collaboration.
To the peritoneal dialysis team of the Central University Hospital of Asturias, for their essential participation in this study.
To Dr. Gonzalo Solís Sánchez for his correct rectifications and to Dr. Pedro Vidau for his enthusiasm in the project and disinterested support.
Please cite this article as: Núñez Moral M, Martínez-Camblor P, Méndez González A, Rodríguez Suárez C, Sánchez Álvarez JE. MUL+DO: índice multicomponente para el diagnóstico rápido de peritonitis en pacientes de diálisis peritoneal. Nefrologia. 2018;38:273–278.