Around 95% of diaphragmatic hernias are posterolateral (Bochdalek), the rest being anterior (Morgagni) or central.1 Morgagni hernias account for 3% of all diaphragmatic hernias2 and mainly occur on the right side.
Clinical presentation is highly variable. Paediatric patients usually have major diaphragmatic defects and, in 80% of cases, recurrent respiratory infection.3 In these cases, associations have been found in up to 80% with congenital heart disease.4 In adults, Morgagni hernias may be the result of minor congenital defects which have not previously been diagnosed or acquired defects, and they develop more slowly.5
In adults they have been associated with different factors such as pregnancy, trauma, obesity, chronic constipation and persistent cough.6 In some cases, they have been associated with the use of peritoneal dialysis (PD) due to an increase in intra-abdominal pressure.7 Although it was thought that most cases in adults were asymptomatic, recent reviews report the presence of some symptoms, in many cases nonspecific or of low severity. Symptoms can include dyspnoea and cough (36%), chest and abdominal discomfort (37%), intestinal obstruction (20%), dysphagia (3%), gastroesophageal reflux or even gastrointestinal bleeding (1%), with only 28% of reported cases being asymptomatic.2
The patient was a 59-year-old female with a previous medical history of advanced chronic kidney disease secondary to chronic pyelonephritis with hypertension and dyslipidaemia. She reported having a pregnancy lasting a number of weeks, but did not reach term, and surgical history of appendectomy and inguinal hernia repair. In January 2017, she was started on incremental PD with an overnight exchange of 2 litres with 1.5% glucose, with no immediate complications.
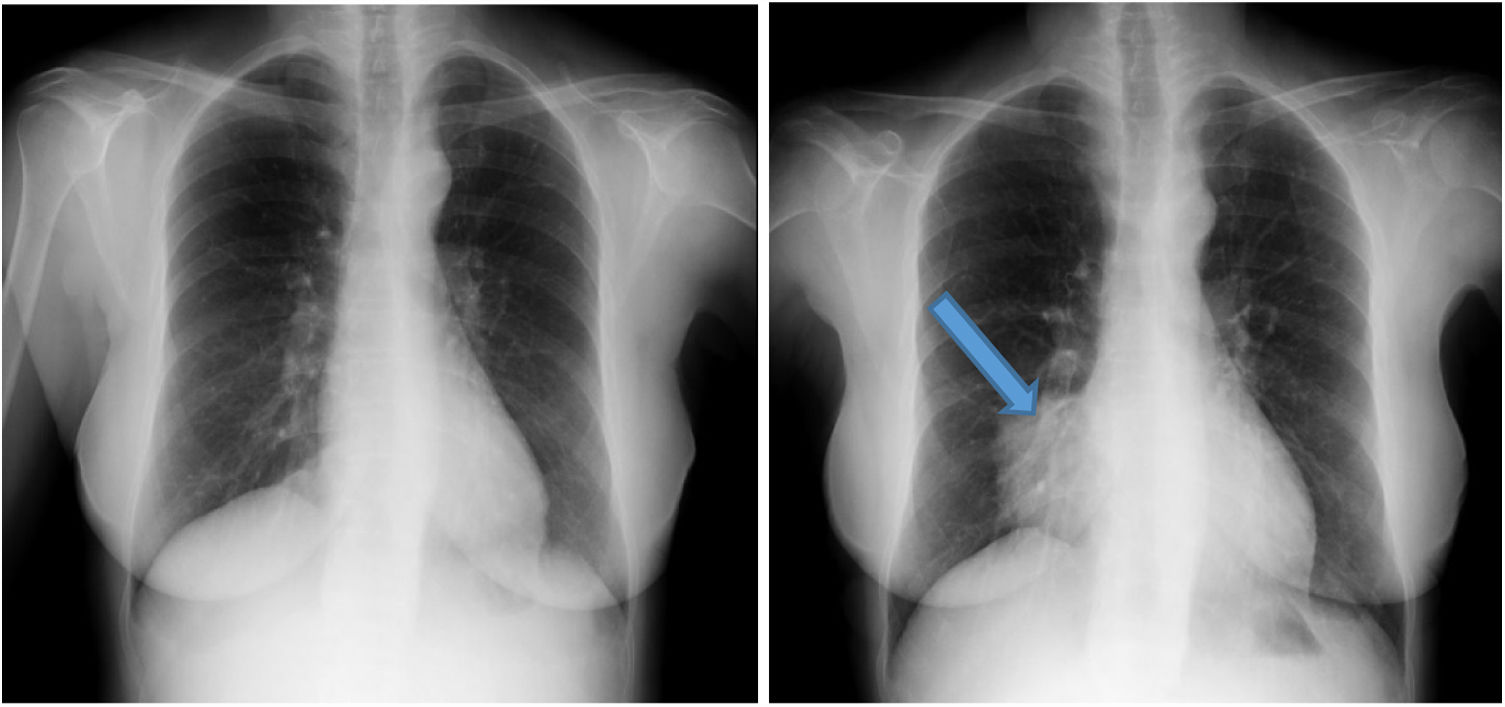
In February 2018, the patient started to experience an increase in her usual dyspepsia, night sweats and slight oedema in the lower limbs, with no associated laboratory abnormalities. In a routine chest X-ray in October 2018 (Fig. 1), an increase in right para-cardiac density was observed, erasing the cardiac silhouette, located in the middle lobe, which was not present in X-rays prior to the start of PD (Fig. 1).
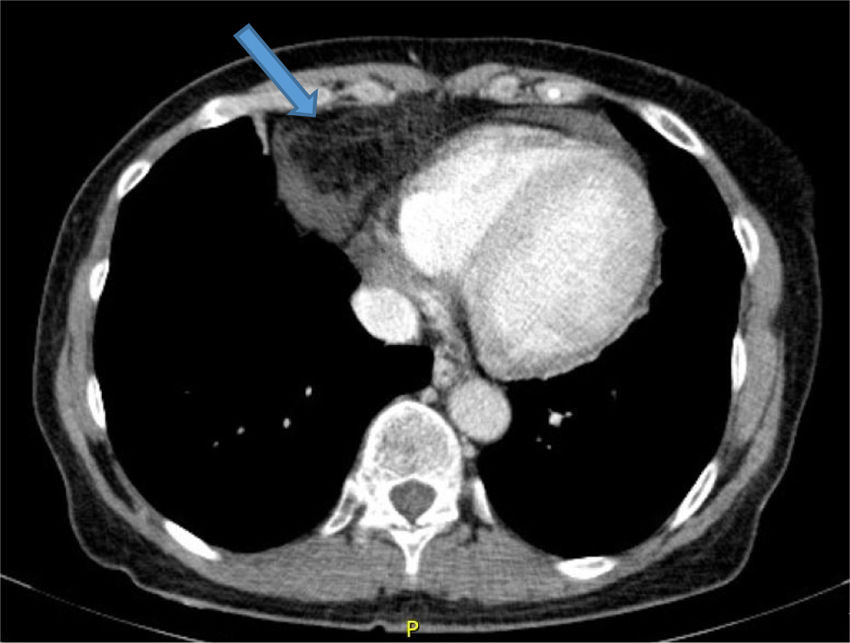
In view of the results of the chest X-ray, an abdominal, pelvic and thoracic CT scan was requested (Fig. 2), which revealed the presence of a Morgagni hernia with visceral fat content and fluid through the hernia orifice. The herniation was causing linear atelectasis at the level of the middle lobe, with no evidence of hilar/mediastinal or axillary lymphadenopathy of significant size or appearance. With the results of the CT scan, a peritoneogram was requested, which ruled out peritoneal fluid leak or pleuroperitoneal communication. The patient was evaluated by general surgery and, given the associated symptoms, the potential complications and the intention to maintain long-term renal replacement therapy with PD, she underwent surgical closure of the hernia defect. Following the surgery, she was temporarily put on haemodialysis. One month after the intervention, and in the absence of associated complications, the patient restarted PD therapy with an overnight exchange. She has gradually been losing residual renal function, for which it has been necessary to increase to 3 daily exchanges, with no complications or new hernias.
We have presented the case of a patient diagnosed with Morgagni hernia after starting PD. Based on the data described, there seems to be a temporal relationship between the start of the technique and the development of the hernia.
Regarding the therapeutic approach for this condition in PD, few cases have been described. In patients with Morgagni hernia not treated with PD, the need for surgery depends on the mode of presentation, and is indicated when there is large intestine present in the hernia sac, or when the symptoms are recurrent or disabling.5
Sastre et al. described the case of a patient with a Morgagni hernia developed during treatment with PD, with symptoms such as constipation, dizziness and cardiac arrhythmia; the decision was made to switch the patient to HD.7
In our case, despite the fact that the symptoms were mild and that the CT scan showed the presence of a hernia sac containing only peritoneal fat, surgical correction was chosen due to the possibility of reintroducing PD according to the patient’s wishes.
This is the first case described in which the option to continue with PD was chosen; in the previously reported cases, whether at the wish of the patient, owing to a lack of experience with similar cases, or due to the caution of the doctors, it was decided to abandon the technique. Our case shows that PD can be a good therapeutic option in patients with diaphragmatic hernias, allowing us to prioritise the patients’ choice and the therapeutic advantages of this renal replacement modality.
Please cite this article as: Santos-Alonso C, Cabrita Da Silva A, Ossorio M, Del Peso G, Maldonado-Martín M, Racionero González P, Selgas Gutiérrez R, Bajo Rubio MA. Hernia de Morgagni en diálisis peritoneal incremental: ¿es posible continuar con la técnica? Nefrologia. 2020;40:685–686.