Membranous glomerulonephritis is a common cause of nephrotic syndrome resulting from immune complex formation on the subepithelial side of the glomerular basement membranes.1 Although most often caused by polytypic deposits, isolated cases with monotypic (single light chain isotype) deposits of immunoglobulins (Ig) have also been reported.
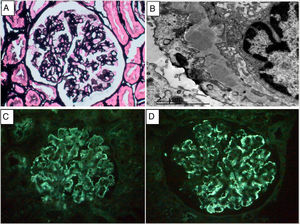
We present the case of a 58-year-old woman with a history of Crohn’s disease diagnosed in 2016, requiring hemicolectomy at onset and treatment with adalimumab. The patient consulted for oedema, nephrotic syndrome was documented with serum Cr of 0.6 mg/dl, proteinuria of 4.2 g/24 h, albumin of 2.7 g/l and sediment with 3–5 RBC/HPF. Complete blood count, liver and thyroid profile were normal. The autoimmunity study showed positive ANA (1:320) and ENA, with complement, anti-PLA2R, anti-DNA, rheumatoid factor, ANCA, cryoglobulins and viral serologies normal. A renal biopsy was performed and 15 glomeruli were obtained, none of them sclerotic. All glomeruli showed mild mesangial matrix expansion without associated cell proliferation. With methenamine silver (Fig. 1) and PAS staining, the glomeruli showed thickened basement membranes, without clear images of double contours. The direct immunofluorescence (DIF) study on the frozen section was negative for the antisera studied (IgG, IgA, IgM, C3, kappa and lambda). The interstitium and vascular structures were normal. Congo red and thioflavin were negative. The immunohistochemical study for C4d and DIF for anti-PLA2R were also both negative. Electron microscopy showed electron-dense deposits in the subepithelial area of the basement membrane and diffuse pedicellar fusion (Fig. 1B). In view of these findings, DIF was repeated on frozen tissue, which was also negative, and the study was completed with DIF on pronase-digested paraffin sections, which documented immune deposits of IgG (+++) and kappa (+++) with a granular pattern (Fig. 1C and 1D), in a subepithelial arrangement. The pathology diagnosis was membranous glomerulonephritis with masked monotypic IgG-kappa deposits. The study was completed with urine and serum immunofixation electrophoresis, which showed no free paraprotein, and a whole body CAT scan was performed with no relevant lesions. After diagnosis, treatment with ACE inhibitors was started, and the patient continued with her background immunosuppressive treatment for inflammatory bowel disease. Four months after onset, the patient experienced a progressive decrease in proteinuria compatible with a spontaneous partial remission, which still persists.
A. Methenamine silver stain. Thickening of capillary walls without proliferative lesions. B. Electron microscopy. Subepithelial electron-dense deposits and pedicellar fusion. C and D. Immunofluorescence on pronase-treated paraffin-embedded tissue: parietal granular deposition of IgG (+++)3 and kappa (+++).4
The term ‘masked deposits’ refers to Ig deposits that show false negative staining by DIF on frozen tissue, but which can be detected when DIF is performed on paraffin-embedded tissue. Nasr et al. were the first authors to identify masked deposits in biopsies from patients with tubular disease associated with light chain deposition.2 The finding of masked deposits has subsequently been documented in other entities, such as glomerulonephritis associated with monoclonal Ig deposition and cryoglobulinaemia.3 In 2014, Larsen et al. first reported a series of 14 cases of glomerulopathy with an optical membranous pattern and evidence of IgG deposits and restriction for kappa in the capillary wall, which they termed “membranous-like glomerulopathy with masked IgG kappa deposits”.4 The deposits showed false negative staining for Ig by conventional DIF, but nevertheless strong positivity for IgG and kappa (but not lambda) by pronase-treated paraffin-embedded DIF. These were relatively young women with an underlying autoimmune substrate, most of whom were positive for antinuclear antibodies.5,6 In all cases, as in our patient, an underlying haematological clonal process that could account for the monotypic immune deposits was ruled out. This light chain restriction in glomerular deposits is rare but does not necessarily indicate an underlying haematological disorder. It has been postulated that in these cases the immune response may actually be polyclonal, with circulating immune complexes, but that only IgG molecules with kappa light chains have the physico-chemical properties that lead to their uptake and deposition in glomeruli. With regard to treatment, there is no standardised guideline for the management of these patients and the spectrum of progression is highly variable. Cases of spontaneous remission have been reported, while other patients had a more aggressive course, progressing to chronic kidney disease despite immunosuppressive treatment.
This case highlights the importance of performing paraffin-embedded DIF studies in cases of glomerulonephritis in which conventional DIF reveals no significant presence of immune deposits. Further studies are needed to investigate the pathophysiological mechanisms leading to this particular form of glomerular involvement.