About 25% of patients with IgA nephropathy (IgAN) progress to stage 5 chronic kidney disease (CKD) after years of evolution. Various tools have been developed in recent years designed to predict which of the patients will had poorer outcomes. The value of circulating galactosyl-deficient IgA1 (Gd-IgA1) has been related to a worse evolution of IgAN in several studies. There are also some publications that relate higher APRIL values with a worse evolution. Recently, a new method has been developed that allows measuring the value of circulating Gd-IgA1 in a simpler way than those previously available. The objective of this study is to analyze the influence of circulating Gd-IgA1, measured by this method, on the progression of IgAN.
Materials and methodsForty-nine patients with a diagnosis of IgAN demonstrated by renal biopsy were selected in our center, without having received prior immunosuppressive treatment, for whom frozen serum was available. The median follow-up was 4 years. Gd-IgA1 was measured by lectin-independent ELISA with the monoclonal antibody KM55 (IgA1 kit Cat. No. 30111694. IBL Int., Hamburg, Germany). Likewise, APRIL levels were also measured in these patients.
Results19 (38.8%) patients reached stage 5 CKD. The fourth quartile of circulating Gd-IgA1 was related to a higher cumulative risk of reaching stage 5 CKD in the Kaplan–Meier analysis (risk at the 5th year 39.4% vs. 24.3%, log rank p=0.019). The Gd-IgA1 value was related to an increased risk of CKD stage 5 (HR 1.147, 95% CI 1.035–1.270, p=0.009), regardless of glomerular filtration rate, proteinuria, the percentage of sclerosed glomeruli and the value of segmental sclerosis. We did not find significant differences in the APRIL values.
ConclusionsThe value of circulating Gd-IgA1 measured by the monoclonal antibody KM55 is related to a worse evolution of patients with IgAN independently of other variables, so it could be included in the study of patients to improve the prediction of the risk of disease progression.
En torno al 25% de los pacientes con nefropatía IgA (NIgA) progresa hacia el estadio 5 de la enfermedad renal crónica tras años de evolución. En los últimos años se han desarrollado diversas herramientas diseñadas para predecir qué pacientes evolucionan peor. El valor de IgA1 galactosil-deficiente (Gd-IgA1) circulante se ha relacionado con una peor evolución de la NIgA en algunos estudios. También hay varios trabajos que relacionan valores más elevados de APRIL con una peor evolución. Recientemente se ha desarrollado un método que permite medir el valor de Gd-IgA1 circulante de una manera más sencilla que los previamente disponibles. El objetivo de este estudio es analizar la influencia de la Gd-IgA1 circulante, medida por este método, en la progresión de la NIgA.
Materiales y métodosSe seleccionaron 49 pacientes con diagnóstico de NIgA demostrado mediante biopsia renal en nuestro centro, sin haber recibido tratamiento inmunosupresor previo, de los que se dispusiera de suero congelado. La mediana de seguimiento fue de cuatro años. Se midió Gd-IgA1 mediante ELISA independiente de lectina con el anticuerpo monoclonal KM55 (IgA1 kit Cat. No 30111694. IBL Int., Hamburgo, Alemania). Así mismo también se midieron los niveles de APRIL en estos pacientes.
Resultados19 (38,8%) pacientes alcanzaron ERC estadio 5. El cuarto cuartil de Gd-IgA1 circulante se relacionaba con un mayor riesgo acumulado de llegar a ERC estadio 5 en el análisis de Kaplan–Meier (riesgo al 5 año 39,4% vs. 24,3%; log rank p=0,019). El valor de Gd-IgA1 se relacionaba con un mayor riesgo de ERC estadio 5 (HR 1,147; IC 95%: 1,035–1,270; p=0,009), independientemente del filtrado glomerular, la proteinuria, el porcentaje de glomérulos esclerosados y el valor de esclerosis segmentaria. No encontramos diferencias significativas en los valores de APRIL.
ConclusionesEl valor de Gd-IgA1 circulante medido mediante el anticuerpo monoclonal KM55 se relaciona con una peor evolución de los pacientes con NIgA independientemente de otras variables, por lo que se podría incluir en el estudio de los pacientes para mejorar la predicción del riesgo de progresión de la enfermedad.
IgA nephropathy (IgA) is the most common primary glomerulonephritis. Initially identified as a benign form, it is known that up to 20–30% of patients reach the final stage of chronic kidney disease (CKD) 20 years after diagnosis.1,2 In some series, with very long-term follow-up, this prognosis is even darker, reaching even end-stage chronic kidney disease (ESRD) in up to 50% of diagnosed patients.3–6 In recent years, various tools have been developed designed to predict the course of IgAN and to know which patients have a greater risk of suffering a worse evolution. Some of these tools use clinical-analytical or histological data or a mixture of both.6–9 Although the efficacy of previously tried treatments to slow the progression of IgAN is controversial, new therapies are being developed that could potentially improve the prognosis of the disease.10–12 In this sense, it would be of the utmost interest to differentiate patients with a high risk of progression, who could benefit from more aggressive treatments, from those with a more benign evolution.13
Since 2001, it has been known that the defect in the galactosylation of the IgA hinge region is the main pathogenic factor that initiates the sequence of events that lead to the development of IgA.14 Several studies relate having a higher value of circulating galactosyl-deficient IgA1 (Gd-IgA1) with a worse evolution of the disease.15–19 However, other studies have not confirmed this relationship.20,21 One of the problems detected when conducting these studies has been the difficulty of reliably and reproducibly measuring circulating Gd-IgA1. The methods used, such as mass spectrometry and the use of lectin-dependent antibodies (Helix aspersa agglutinin) for their reactivity, are difficult to carry out and are not available in many research and healthcare centers. In 2015, Yasutake et al. developed a monoclonal antibody KM55 that does not depend on lectin and that makes the measurement of Gd-IgA1, both circulating and on tissue, easier and more reproducible.22 Previous studies with this antibody in patients with IgAN have shown that circulating Gd-IgA1 is related to histological findings, glomerular Gd-IgA1 deposition, and renal function and proteinuria at the time of biopsy, although not consistently.23–26 No studies have been conducted that relate the measurement of Gd-IgA1 by this method with the evolution of long-term renal function in a non-Asian population.
On the other hand, it is known that the proliferation inducing ligand ("A Proliferation Inducing Ligand", APRIL) participates in the pathogenesis of NIgA by increasing the production of Gd-IgA1 in the mucosa.27,28 The aim of our study was to analyze the relationship between circulating Gd-IgA1 and APRIL in humans at the time of the diagnostic biopsy of IgAN and its influence on the long-term progression of nephropathy.
Material and methodRenal biopsies performed between 1990 and 2019 diagnosed with IgA nephropathy classified with the Oxford criteria were retrospectively selected, from which a sample of serum extracted and frozen was available before the biopsy and before starting immunosuppressive treatment and with complete monitoring of the patient throughout their evolution. As a comparison group, the kidney biopsies performed between March/2015 and December/2018 were selected for suspected glomerular process (11 focal and segmental hyalinosis, five interstitial nephritis, four membranous GN, four RPGN, three TMA, three amyloidosis, two membranoproliferative, two diabetic nephropathy, two nephroangiosclerosis and one postinfectious GN). Those biopsies for which serum was not available before the biopsy, and also those with a diagnosis of lupus nephritis were excluded because it was a possible confounding factor when analyzing the Gd-IgA and APRIL values. No patient received immunosuppressive treatment before the biopsy. Healthy patients were not included as a second control group. Gd-IgA1 was measured by lectin-independent ELISA with monoclonal antibody KM55 (IgA1 kit Cat. No. 30111694. IBL Int., Hamburg, Germany). The determination of APRIL was performed using the RYD-DY884B Human APRIL/TNFSF13 DuoSet ELISA kit (R&D Systems, Inc., Minneapolis, Minn., USA).
Relevant demographic data were collected from the clinical history, as well as the following clinical and biochemical parameters in blood and urine: age, gender, systolic and diastolic blood pressure (SBP), hemoglobinuria (result expressed semi-quantitatively, in crosses, by means of the elemental or systematic study of urine by automated test strips), creatinine, glomerular filtration rate (GFR) estimated by CKD-EPI, uric acid, albumin, hemoglobin and 24-h proteinuria at the time of biopsy. Stage 5 CKD was defined when patients achieved a glomerular filtration rate below 15mL/min, started dialysis, or underwent transplantation. The moment when the glomerular filtration rate dropped below 30mL/min was also recorded. Regarding the pathological data, the number of sclerosed glomeruli and the variables of the MEST classification were collected, including the percentage of crescents.
On the other hand, the risk of progression at five years was calculated using two different free access online tools: the online IgA Nephropathy Progression Calculator (IgANPC) accessed through the web http://www.columbiamedicine.org/divisions/gharavi/calc.progression.php and the International Risk-Prediction Tool in IgA Nephropathy (IRPT-IgAN) accessed through the website https://qxmd.com/calculate/calculator_499/international-igan-prediction-tool. In the case of IgANPC, four parameters determined at the time of the renal biopsy and obtained from the patient's medical history and electronic records are used: GFR, serum hemoglobin expressed in g/dL, serum albumin expressed in g/dL and SBP expressed in mmHg. To calculate the five-year risk of reaching stage 5 CKD using the other prognostic calculator, the IRPT-IgAN, five clinical parameters were collected such as blood pressure (BP), age, race, use of angiotensin converting enzyme inhibitors (ACEI) or angiotensin II receptor antagonists (AIIRAs), use of immunosuppression prior to biopsy, laboratory tests such as estimated glomerular filtration rate (eGFR) and proteinuria at the time of biopsy, and also the histological data obtained from the MEST-C.
Statistic analysisThe difference between the IgA and APRIL values between the different groups was compared using the Mann–Whitney U test for dichotomous variables and using the Kruskal–Wallis test for variables with more than two categories. The discriminative ability of Gd-IgA1 and APRIL to diagnose IgAN was calculated using the ROC curve. The relationship between Gd-IgA1 and the rest of continuous variables was analyzed using Spearman's correlation. The relationship between the different variables and the risk of stage 5 CKD was analyzed using univariate and multivariate Cox regression.
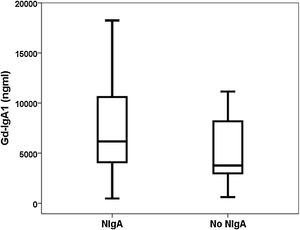
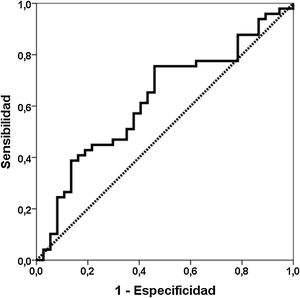
ResultsDiscrimination ability of Gd-IgA1 and APRILForty-nine patients with a diagnosis of IgA nephropathy were compared with 37 biopsied patients with other diagnoses (Table 1). The Gd-IgA1 value was 6170 (IQR 7623) ng/mL in patients with IgAN and 3823 (IQR 5353) ng/mL in patients with other diagnoses (p=0.047) (Fig. 1). The APRIL value was 1149 (IQR 1189) pg/mL in patients with IgAN and 1083 (IQR 916) pg/mL in patients with other diagnoses (p=0.239). The area under the ROC curve (AUC-ROC) of Gd-IgA1 to predict IgA was 0.625 (95% CI: 0.506–0.745; p=0.047) (Fig. 2). The optimal cut-off point was 3900ng/mL, with a sensitivity of 75.5%, a specificity of 54.1%, a positive predictive value of 68.5%, and a negative predictive value of 62.5%. The AUC-ROC of APRIL to discriminate the presence of IgAN in the renal biopsy was 0.575 (95% CI: 0.454–0.697; p=0.239).
Patient characteristics.
| IgAN (n=49) | No IgAN (n=37) | p | |
|---|---|---|---|
| Age (years) | 51.3±15.9 | 59.5±15.2 | 0.019 |
| Male gender | 79.6% | 48.6% | 0.003 |
| Creatinine (mg/dl) | 2.1±1.3 | 1.9±1.2 | 0.496 |
| Estimated GFR (ml/min/1.73m2) | 49±31 | 46±27 | 0.612 |
| Proteinuria (g/day) | 3.0±2.8 | 3.6±3.8 | 0.316 |
| Hemoglobinuria (+) | 3±1 | 1±1 | <0.001 |
| Systolic blood pressure (mmHg) | 142±21 | 131±18 | 0.027 |
| Diastolic blood pressure (mmHg) | 82±13 | 76±12 | 0.045 |
| Uric acid (mg/dl) | 7.2±2.2 | 7.0±2.5 | 0.655 |
| % Sclerosed glomeruli | 3.2±5.1 | – | – |
| M (0/1) | 8/41 | – | – |
| E (0/1) | 24/25 | – | – |
| S (0/1) | 24/25 | – | – |
| T (0/1/2) | 23/20/6 | – | – |
| % crescent | 14.9±23.6 | – | – |
| Risk calculated at 5 years IgANPC | 35±33 | – | – |
| Risk calculated at 5 years IRPT-IgAN | 25±20 | – | – |
| SRAA lock | 31 (63%) | ||
| Use of Steroids | 21 (43%) | ||
| Duration of treatment Steroids (months) | 6.8±11.5 | ||
| Any immunosuppressant | 13 (27%) |
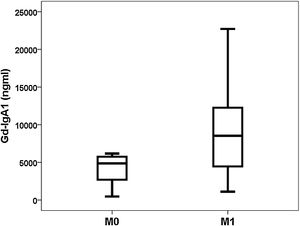
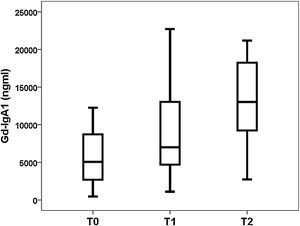
Gd-IgA1 correlated with M (rho=0.308; p=0.031) and T (rho=0.377; p=0.008), but it did not correlate with the rest of the histological parameters (percentage of sclerosed glomeruli, percentage of crescents, E and S). Regarding non-histological variables, Gd-IgA1 correlated with renal function at the time of biopsy (rho=−0.308; p=0.034) and with uric acid value (rho=0.322; p=0.029), but It did not correlate with the patient's age (rho=0.020; p=0.890), with blood pressure (systolic rho=−0.115; p=0.432; diastolic rho=−0.225; p=0.120), with hemoglobinuria (rho=0.062; p=0.671), with proteinuria (rho=0.045; p=0.761) or with the estimated risk at five years (rho=0.182; p=0.210). On the contrary, it did correlate with the risk at five years calculated by IRPT-IgAN (rho=0.365; p=0.010). The Gd-IgA1 values were not different depending on the sex of the patient (female 5493 and IQR 6529ng/mL vs. male 6643 and IQR 8740ng/mL; p=0.650), but they were higher in patients with more affectation histological in M (M0 4.866 and IQR 3176ng/mL vs. M1 8528 and IQR 8277ng/mL; p=0.032) (Fig. 3) and in T (T0 5075 and IQR 6266ng/mL vs. T1 7013 and IQR 8468ng/mL vs. T2 13,025 and IQR 11,358ng/mL; p=0.032) (Fig. 4). The circulating APRIL value did not correlate with circulating Gd-IgA1 or with any of the clinical, analytical or histological parameters analyzed.
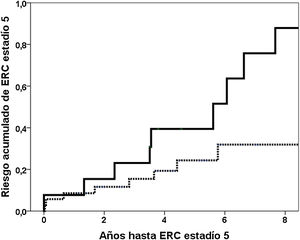
The median follow-up to stage 5 or the last follow-up was 4.0 years (25th percentile: 2.0; 75th percentile: 5.9 years). 19 (38.8%) patients reached stage 5 CKD and 22 (44.9%) reached stage 4. The variables related to the risk of stage 5 CKD are shown in Table 2. In model 1 including the significant variables Gd-IgA1, APRIL, glomerular filtration, proteinuria, % sclerosed glomeruli and segmental glomerular sclerosis (S), the value of GD-IgA1 was independently associated with an increased risk of stage 5 CKD. The fourth quartile of Gd-Circulating IgA1 was related to a higher cumulative risk of reaching stage 5 CKD in the Kaplan–Meier analysis (risk at 5° year 39.4% vs. 24.3%; log rank p=0.019) (Fig. 5). In the second model, encompassing the clinical-analytical data in the risk variable estimated at five years, the value of circulating Gd-IgA1 was independently related to a higher risk of stage 5 CKD. In the same models, substituting the continuous variable Gd-IgA1 for dichotomous variables (fourth quartile, third tertile) lost statistical significance.
Variables related to the risk of reaching stage 5 CKD.
| Univari ante | Multivariate | |||||||
|---|---|---|---|---|---|---|---|---|
| HR (IC) | p | HR (IC) model 1 | p | HR (IC) model 2 | p | HR (IC) Model 3 | p | |
| Gd-IgA1 (μg/mL) | 1092 (1024–1165) | 0.007 | 1147 (1035–1270) | 0.009 | 1141 (1036–1257) | 0.008 | 1102 (1000–1214) | 0.049 |
| Fourth Quartile Gd-IgA1 | 2930 (1149– 7476) | 0.024 | – | – | ||||
| APRIL (ng/mL) | 2086 (1114–3906) | 0.022 | 1647 (0.632–4.297) | 0.307 | 2.113 (0.857–5.213) | 0.104 | 2025 (0.824–4.979) | 0.124 |
| Estimated glomerular filtration rate (ml/min/1.73m2) | 0.956 (0.931–0.982) | 0.001 | 0.932 (0.894–0.971) | 0.001 | – | – | ||
| Hemoglobinuria | 0.988 (0.680–1.434) | 0.949 | – | – | – | – | ||
| 24-h proteinuria (grams) | 1317 (1118–1551) | 0.001 | 1410 (1077–1845) | 0.012 | – | – | ||
| Age | 1.026 (0.993–1.060) | 0.124 | – | – | – | – | ||
| Risk Calculated 5 years IgANPC | 1024 (1010–1038) | 0.001 | – | – | 1.014 (0.994–1.034) | 0.180 | – | – |
| Risk Calculated 5 years IRPT-IgAN | 1059 (1032–1086) | <0.001 | 1041 (1003–1080) | 0.032 | ||||
| % sclerosed glomeruli | 1.124 (1.046–1.209) | 0.002 | 0.978 (0.884–1.082) | 0.663 | 1.064 (0.967–1.172) | 0.204 | 1.007 (0.903–1.123) | 0.897 |
| M | 2.483 (0.325–18.993) | 0.381 | – | – | – | – | – | – |
| AND | 1.684 (0.619–4.583) | 0.307 | – | – | – | – | – | – |
| S | 6678 (1811–24,622) | 0.004 | 6678 (1811–24,622) | 0.002 | 5857 (1180–29,075) | 0.031 | 6015 (1209–29,936) | 0.028 |
| T | 1.891 (0.989–3.616) | 0.054 | – | – | – | – | – | – |
| % crescent | 1.014 (0.996–1.031) | 0.127 | – | – | – | – | – | – |
| SRAA lock | 0.887 (0.336–2.342) | 0.809 | ||||||
| Steroid Use (Yes) | 1.419 (0.525–3.838) | 0.491 | ||||||
| Duration of treatment Steroids (months) | 0.976 (0.930–1.024) | 0.323 | ||||||
| Any immunosuppressant | 0.569 (0.162–2.005) | 0.569 | ||||||
In our group of renal biopsies, we found that circulating Gd-IgA1 was significantly higher in patients with IgAN than in patients with other diagnoses in the renal biopsy. Different studies carried out both measuring Gd-IgA1 with lectin-dependent and non-dependent antibodies (KM55) have reported that patients with IgAN have higher levels of Gd-IgA1 compared with a group of healthy controls and with other patients with biopsy due to kidney disease.11,23,25,26,29–35 However, other authors have not found these differences.36 Similarly, the ability of Gd-IgA1 to discriminate which patients have IgAN demonstrated in renal biopsy has ranged from 0.749 to 0.902.26,29,35 This low discrimination capacity, less than 70%, between patients with and without IgAN observed in our study, suggests that the clinical utility of the test is scarce and that it should not be incorporated into the usual routine as a diagnostic criterion. The sweet spot had a sensitivity of 76%, but the specificity was only 54%. Illustratively, in Fig. 1 it can be seen that Gd-IgA1 values were elevated in many patients with IgAN, but also that many IgAN patients had indistinguishable Gd-IgA1 values from those without IgA. This overlap in circulating Gd-IgA1 values between patients with and without IgAN has also been previously highlighted by other authors.26,35,37
Relationship between circulating Gd-IgA1 and histological findingsInterestingly, we found that the value of Gd-IgA1 correlated significantly with two of the histological parameters included in the classification of Oxford on which it has demonstrated its relationship with the prognosis of the disease in July. In our study, Gd-IgA1 was higher in patients with more mesangial hypercellularity and more tubular atrophy. In the case of tubular atrophy, the more severe the lesion (T2>T1>T0), the circulating Gd-IgA1 levels were higher. On the one hand, it is known that a higher level of circulating Gd-IgA1 correlates with a higher mesangial deposit of Gd-IgA1.23–25 In fact, the exposure of mesangial cells in cultures to Gd-IgA1 is related to an increase in the secretion of a marker of mesangial inflammatory response such as monocyte chemotactic protein 1 (MCP-1).38 It could be speculated that this increase in local deposit would increase the lesion on the mesangium and increase its cellularity, for which the level of circulating Gd-IgA1 should be correlated with greater mesangial hypercellularity. On the other hand, chronic damage that reflects tubular and interstitial involvement could be an expression of the greater sustained kidney damage produced by circulating and deposited Gd-IgA1. However, other authors have reported that circulating Gd-IgA1 is not related to histological alterations23,26 or is related to other sections of the Oxford classification.24,38 For example, Nguyen et al. found a correlation between Gd-IgA1 and segmental glomerulosclerosis "S" and tubular atrophy "T" in a study of 33 patients with IgAN.38 So far the series analyzed have been too small to be able to draw definitive conclusions about the relationship between Gd-IgA1 and MEST-C.
Relationship between circulating Gd-IgA1 and clinical and analytical variablesCirculating Gd-IgA1 was not different according to the sex of the patient nor was it related to age, a fact already observed by other authors.23 A relationship was observed between elevated Gd-IgA1 levels and worse kidney function and higher uric acid levels. This relationship had already been observed previously,23–25 although not in all studies,26,29,38 and could be explained by the correlation with chronic histological lesion at the tubular level. The lack of relationship with the degree of hemoglobinuria, already demonstrated by Zhang et al.,23 and with the degree of proteinuria23,38 it could be due to the fact that these appear as a consequence of the entire cascade of events after the first "hit " that Gd-IgA1 represents, such as inflammation, complement activation and podocyte damage.39
Relationship between circulating Gd-IgA1 and CKD progressionThe most relevant finding of our study was the negative influence that elevated circulating levels of Gd-IgA1 have on renal evolution. In the univariate study, patients with IgAN in the upper quartile of Gd-IgA1 had almost three times the risk of reaching stage 5 CKD (Fig. 5). As a continuous variable, Gd-IgA1 was also related to a worse evolution, independently of other clinical and histological variables. Despite having carried out a study with a limited number of patients, using three validated prognostic methods such as the Oxford MEST-C histological method and the clinical-analytical prediction tools IgA nephropathy progression calculator (IgANPC) and Risk-Prediction Tool in IgA Nephropathy (IRPT-IgAN), Gd-IgA1 was able to provide information on the prognosis of renal function independently of them. In addition, Gd-IgA is related to the IRPT-IgAN prognostic tool, which supports that there is a relationship with clinical characteristics with prognostic value in IgA nephropathy. Also important is this association between IRPT-IgAN and a pathogenic marker such as Gd-IgA, not observed with IgANPC.
Using technically more complex Gd-IgA1 detection methods with lectin, other authors had described this relationship between elevated levels of circulating Gd-IgA1 and greater progression of CKD.15–19 Thus, Maixnerova et al. reported in 91 patients from the Czech Republic a discriminatory capacity of 84% regarding which patients were going to progress the deterioration of renal function using the initial glomerular filtration, the MEST data and the circulating Gd-IgA1.40 In the largest study conducted, Chen et al. observed in 1210 patients with IgAN followed for a median of 43 months a non-linear relationship between circulating levels of Gd-IgA1 and the risk of progression, also independently of the other variables.19 Already using a lectin-independent method with KM55 to measure Gd-IgA1, Bagchi et al. they did not find a significant relationship between Gd-IgA1 levels and CKD progression in 136 Indian patients with IgAN.26 Our study is the first to use this methodology to analyze the relationship between Gd-IgA1 and the progression of IgAN in a European population. Although the sample of patients with IgAN is small, the patients were followed for a long time, with a median of 48 months, which allows us to draw conclusions about the risk of progression of IgAN.
Specific treatment of IgAN with steroids and other immunosuppressants, independent of the antihypertensive and proteinuria ACEI/ARA2, is partially effective in limiting the progression of renal disease and have side effects that limit their application.10 Regarding the relationship between immunosuppressive treatment and serum IgA1-Gd levels, a study published in recent years has observed that steroid treatment reduces IgA1Gd levels,41 while the use of Rituximab does not decrease levels. of IgA1-Gd nor those of IgG anti-Gd-IgA, which could explain their lack of efficacy in treating this nephropathy.42 For the use of standardized treatments for this disease, as well as to be able to use the new treatments that have been developed, it is of the utmost interest to have tools that allow identifying patients with a higher risk of progression. Various groups have developed these prognostic assessment scales that included histological variables such as the Oxford classification or clinical-analytical variables such as the IgANPC.6,7 Recently the “International IgA Nephropathy Network” group developed a risk assessment scale incorporating both the Oxford histological variables and clinical-analytical variables with which a prediction of over 80% of the risk of a 50% decrease in the FG or reaching stage 5 ERC.9 In our study, the circulating Gd-IgA1 value provided information with prognostic utility independent of the histological and clinical-analytical variables.
Given that the new IRPT-IgAN tool seems more powerful than IgANPC, in our study the IgAGd loses statistical value and remains on the edge of significance, but even so it remains a variable related to the risk of poor evolution of nephropathy IgA, regardless of a marker as robust and as demonstrated in multicenter studies as IRPT-IgAN.
If its usefulness is demonstrated in studies with a larger number of patients, adding the value of circulating Gd-IgA1 measured by a simple and reproducible technology, such as the KM55 antibody, to these risk prediction scales could contribute to increasing their prognostic precision.
Role of APRIL in the diagnosis of IgANOn the other hand, in our study, APRIL was not useful for the diagnosis of IgAN, nor was it correlated with the value of circulating Gd-IgA1 or with other clinical or laboratory variables. An increased risk of reaching stage 5 was only observed in patients with higher values of circulating APRIL in the univariate analysis.
Different types of studies support the role of APRIL in the pathogenesis of IgAN. Some APRIL polymorphisms have been associated with a higher risk of IgAN, with higher proteinuria, poorer kidney function, and a higher risk of progression to stage 5.43–46 APRIL is produced mainly in the epithelial, dendritic and myeloid cells of the mucosa and favors the maturation and proliferation of B cells.47,48 The microbial exposure in the mucosa induces the production of TLR9 that increases the synthesis and release of APRIL by dendritic cells and the consequent increase in the production of IgA, mainly Gd-IgA1, which will be the first step for the appearance of IgA.27,28,47 Human studies have shown that circulating APRIL levels were higher in patients with IgAN and were associated with Gd-IgA1 levels, poorer histology, poorer kidney function, and proteinuria. The disagreement with our findings may be due to the high number of patients with IgAN included in these studies (637, 1000, 99, 166, respectively).45,46,49,50 On the other hand, our group had observed that the risk of recurrence of IgAN after kidney transplantation was more related to the maintenance of high levels of APRIL throughout the post-transplant time than to an isolated point value, as has been carried out carried out in this study, so the partially contradictory results cannot be extrapolated.51
To conclude, the development of the new monoclonal antibody KM55 has provided an affordable and reproducible methodology to measure circulating Gd-igA1. Although its usefulness as a non-invasive marker of IgAN is limited, circulating Gd-IgA1 is related, independently of other variables, with the risk of progression of IgAN to the end stage of CKD. If this relationship is confirmed in larger studies, it should be incorporated into the rest of the available predictive tools, since the current trend to detect those patients with a worse prognosis in this entity is to associate different clinical, analytical and pathological markers in a unique predictive tool.
Conflict of interestsThe authors declare that they have no conflict of interest.
Please cite this article as: Martín-Penagos L, Fernández-Fresnedo G, Benito-Hernández A, Mazón J, de Cos M, Oviedo MV, et al. La determinación de IgA1 galactosil deficiente mediante el anticuerpo monoclonal KM55 contribuye a predecir a los pacientes con nefropatía IgA con alto riesgo de progresión a largo plazo. Nefrologia. 2021;41:311–320.