In nephrotic syndrome, increased podocyturia accompanies pathologic proteinuria. The therapeutic regimen with enalapril, losartan and amiloride could reduce both variables.
ObjetivesEvaluate the anti-proteinuric effect of 2 non-immunological therapeutic regimens, the quantitative relationship between podocyturia and proteinuria.
Material and methodsWe included children aged 4–12 years with corticoresistant nephrotic syndrome, using 2 different schemes: group A, enalapril + losartan, and group B, enalapril + losartan + amiloride.
ResultsIn group A, 17 patients completed the study, the initial mean proteinuria was 39 mg/m2/h and mean proteinuria at the end was 24 mg/m2/h, while in group B 14 patients were treated and the initial average proteinuria was 36 mg/m2/h and the end average proteinuria was 13 mg/m2/h. The paired T test showed significant differences in the decrease in proteinuria, for patients in group B without variation in podocyturia. The 2 factors associated with an increase in proteinuria were podocyturia and the time elapsed from the diagnosis of cortico-resistant nephrotic syndrome to the start of treatment anti-proteinuric.
ConclusionsThe use of amiloride decreased proteinuria, without significantly modifying podocyturia; we did not observe a positive relationship between both variables.
En el síndrome nefrótico, la podocituria aumentada acompaña a la proteinuria patológica. El tratamiento con enalapril, losartán y amiloride podría reducir ambas variables.
ObjetivosEvaluamos el efecto antiproteinúrico de 2 esquemas terapéuticos no inmunológicos, la relación cuantitativa entre la podocituria y la proteinuria, y las variables de riesgo asociadas con la proteinuria.
Material y métodosIncluimos niños de 4 a 12 años de edad con síndrome nefrótico corticorresistente, utilizando 2 esquemas terapéuticos distintos: grupo A, enalapril + losartán, y grupo B, enalapril + losartán + amiloride.
ResultadosEn el grupo A finalizaron el estudio 17 pacientes, la proteinuria promedio inicial fue de 39 mg/m2/h y la proteinuria media al finalizar fue de 24 mg/m2/h, en tanto que en el grupo B tratamos a 14 pacientes, la proteinuria promedio inicial fue de 36 mg/m2/h y la proteinuria promedio final de 13 mg/m2/h, y si bien ningún paciente redujo su proteinuria a valores fisiológicos, tanto la prueba t apareada (11,5, p < 0,001) como la prueba ordinal logarítmica (log-rank, χ2: 4, p < 0,05) mostraron diferencias significativas en la disminución de la proteinuria, para los pacientes del grupo B frente a los pacientes del grupo A. En ninguno de los 2 grupos hubo una variación significativa de la podocituria durante el estudio, y los 2 factores asociados con el incremento de la proteinuria en el modelo de riesgos proporcionales fueron la podocituria y el tiempo transcurrido desde el diagnóstico de síndrome nefrótico corticorresistente primario hasta el inicio del tratamiento antiproteinúrico. En ningún paciente se observaron efectos adversos de los tratamientos empleados.
ConclusionesEl uso de amiloride disminuyó la proteinuria, sin modificar significativamente la podocituria; no observamos una relación positiva entre ambas variables.
Persistent proteinuria leads to a gradual decline in glomerular filtration rate (GFR) and is a marker of progressive kidney injury; reduction of proteinuria appears to be associated with preserved kidney function.
Traditionally, although non-immunological therapeutic regimens with angiotensin-converting enzyme inhibitors and angiotensin-2 receptor blockers have been implemented to reduce urine protein levels, for some time successful experiences have been documented with the addition to the above nephroprotective regimen of a urokinase-type plasminogen activator receptor inhibitor such as amiloride, which reduces podocyte damage and could therefore lessen proteinuria.
In nephrotic syndrome, podocyte detachment caused by pathological contraction appears to precede the appearance of proteinuria, therefore the grounds for this study are to examine the action of amiloride on podocyturia and proteinuria in paediatric patients with primary steroid-resistant nephrotic syndrome (SRNS).
Objectives- 1.
To evaluate the antiproteinuric effect of two non-immunological therapeutic regimens.
- 2.
To evaluate the quantitative relationship between podocyturia and proteinuria.
- 3.
To evaluate the risk factors associated with proteinuria.
From March 2016 to March 2020, paediatric patients with a history of primary SRNS from the public and private healthcare systems were selected for a controlled, randomised, paired, unblinded clinical trial in children 4–12 years of age, all with renal biopsies and without genetic studies.
We considered:
- a.
Inclusion criteria: we enrolled patients with proteinuria >4 mg/m2/h, mean blood pressure readings below the 95th percentile for their age, gender, weight and height, with no specific immunosuppressant treatments for at least 6 months prior and GFR above 90 ml/min/1.73 m2. Medical informed consent was required.
- b.
Exclusion criteria: patients were excluded if they had intolerance or allergic reactions to the indicated treatments, a single kidney, renovascular hypertension, dysnatraemia or active infectious and/or oncological disease.
- c.
Discontinuation criteria: patients were withdrawn from the study if they presented a persistent reduction in GFR of more than 30% of the initial value during follow-up in at least two consecutive controls, hyperkalaemia (>5.5 mEq/l) that could not be corrected with usual treatment, symptomatic hypotension, diuretic rhythm reduced to less than 1 ml/kg/h, symptomatic hyponatraemia or if they did not attend at least 80% of the established appointments.
- d.
Treatments: the population was randomised to two groups: group A received enalapril and losartan, while group B was treated with enalapril, losartan and amiloride. A low sodium diet was maintained in both groups.
- e.
Follow-up: clinical and laboratory follow-up was conducted in the entire population with SRNS from the start of treatment, then every three months. At each visit, weight, height and blood pressure were measured, with the values considered normal based on percentiles for weight and age and blood pressure measured using the formula: 1/3 systolic blood pressure + 2/3 diastolic blood pressure.1
The laboratory controls performed were: protein electrophoresis. 24 h proteinuria (expressed in mg/m2/h), serum creatinine values (calculating creatinine clearance using the Schwartz method with Barratt’s constant corresponding to sex and age, expressed in ml/min/1.73 m2), complete blood count, serum concentrations of urea, uric acid, electrolytes, lipids and concentration of electrolytes in urine (to estimate dietary sodium intake we calculated that each gram of salt corresponds to 17 mEq/l of urinary sodium).
Podocyturia was measured at two points: before starting treatment and at the end of treatment. It was quantified using the same urine collected for laboratory studies (urine collected over 24 h); the samples were processed within one hour of collection, using a volume of 10 ml to which was added 1 ml of 10% buffered formalin in phosphate buffer (pH 7.2–7.4). An indirect immunofluorescence technique in a humidified chamber was used and the samples were observed under a Nikon Eclipse E200 epi-fluorescence microscope. The number of synaptopodin positive cells was quantified in ten 200× fields for each double-blinded sample. Podocyturia was expressed as number of podocytes per 100 mg of creatininuria.2
The duration of the study was 24 months.
- f.
Variables assessed: we identified podocyturia and proteinuria as dependent variables; and time elapsed from SRNS diagnosis to the last podocyturia and proteinuria measurement, and doses of enalapril, losartan and amiloride administered as independent variables.
- g.
Statistical analysis: we calculated a sample size of 30 patients, and allowing for a loss of 20%, sought to enrol a population of 36 patients per group, using simple 1:1 randomisation.
We used relative risk, absolute risk reduction and number of patients required to assess changes in proteinuria in both groups. Student’s t test was used to determine whether there was a significant difference with regard to proteinuria values. To assess the reduction in the number of patients with heavy proteinuria at different points in the study, we used the log-rank test.
The correlation between proteinuria and podocyturia (quantitative variables) was established with Spearman’s coefficient and finally, we used the Cox proportional hazard coefficient to assess the association between the potential risk factors (age, time elapsed from SRNS diagnosis to start of non-immunological anti-proteinuric treatment, podocyturia and GFR) and magnitude of proteinuria.
The data are presented as means and standard deviation and a p < 0.05 was accepted as significant.
Definitions:
Physiological proteinuria: value in urine less than or equal to 4 mg/m2/h.3
Significant proteinuria: values in urine greater than 4 mg/m2/h and up to 40 mg/m2/h.3
Heavy proteinuria: value in urine greater than 40 mg/m2/h or a proteinuria/creatininuria ratio in a single urine sample greater than 3.3
Primary nephrotic syndrome: heavy proteinuria with hypoalbuminaemia (defined as albumin ≤2.5 g/dl), generally associated with oedema and hypercholesterolaemia.4
SRNS: corresponds to a lack of remission of proteinuria when treated with: (a) prednisone 2 mg/kg/day or 60 mg/m²/day (maximum dose, 60 mg) on consecutive days for 4–6 weeks as one daily dose in the morning between 8 and 10 a.m., followed by prednisone 1.5 mg/kg or 40 mg/m² on alternate days for 4–6 weeks; (b) prednisone 60 mg/m²/day or 2 mg/kg/day for 4–6 weeks on consecutive days plus three pulses of methylprednisolone at 10 mg/kg/dose.4
The study, approved by the hospital’s Independent Ethics Committee, adhered to the Declaration of Helsinki and the principles described in the “Good Clinical Practice Guidelines” of the International Council for Harmonisation’s harmonised tripartite guideline.
ResultsThirty six patients with SRNS were enrolled in the protocol, nine of them girls, with a mean age of 11 years (r = 4.1–14.2). Thirty one patients assigned to the two groups (group A and group B) completed the study (Table 1).
Description of patients with steroid-resistant nephrotic syndrome.
| Group A patients | Gender | Age (years) | Time elapsed from start of steroid treatment to start of anti-proteinuric treatment (months) | Histological diagnosis | Immunosuppressants used |
|---|---|---|---|---|---|
| 1 | Male | 7.5 | 28 | FSGS | MMF, CsA |
| 2 | Male | 6.2 | 26 | FSGS | CsA, MMF |
| 3 | Male | 7.5 | 28 | MP | CsA |
| 4 | Female | 8.5 | 24 | MP | CP, CsA |
| 5 | Male | 11.1 | 29 | FSGS | CP, CsA |
| 6 | Male | 11.9 | 33 | FSGS | CP, MMF, CsA |
| 7 | Male | 8.5 | 27 | MCD | CsA |
| 8 | Female | 5.7 | 26 | FSGS | CsA, MMF |
| 9 | Female | 9.9 | 22 | FSGS | CsA |
| 10 | Male | 5.4 | 29 | FSGS | CP, CsA |
| 11 | Male | 7.9 | 33 | MP | CP, CsA |
| 12 | Male | 8.5 | 28 | MCD | CP, CsA |
| 13 | Male | 5.8 | 22 | MP | CP, CsA |
| 14 | Male | 6.8 | 31 | FSGS | CP, MMF, CsA |
| 15 | Female | 5.3 | 27 | MP | CsA |
| 16 | Male | 5.1 | 29 | MP | MMF, CsA |
| 17 | Male | 10.5 | 26 | FSGS | CsA |
| Group B patients | Gender | Age (years) | Time elapsed from start of steroid treatment to start of anti-proteinuric treatment (months) | Histological diagnosis | Immunosuppressants used |
|---|---|---|---|---|---|
| 1 | Male | 5.3 | 24 | MP | CP, CsA |
| 2 | Male | 7.2 | 28 | MP | MMF, CsA |
| 3 | Female | 10 | 22 | MP | CsA, MMF |
| 4 | Female | 10.4 | 26 | FSGS | CsA |
| 5 | Male | 6.6 | 33 | MCD | CP, CsA |
| 6 | Male | 8.6 | 26 | MP | CP, CsA |
| 7 | Male | 8.3 | 26 | FSGS | CP, MMF, CsA |
| 8 | Male | 5.7 | 29 | FSGS | CsA |
| 9 | Female | 4.9 | 25 | MP | CP, CsA |
| 10 | Male | 9.2 | 24 | MCD | MMF, CsA |
| 11 | Male | 11.3 | 26 | MP | MMF, CsA |
| 12 | Female | 7.9 | 26 | MP | MMF, CP |
| 13 | Female | 10.4 | 25 | FSGS | CP, CsA, MMF |
| 14 | Male | 9.3 | 22 | MP | CP, CsA |
CP: cyclophosphamide; CsA: cyclosporin; EN: enalapril; FSGS: focal segmental glomerulosclerosis; LO: losartan; MCD: minimal change disease; MMF: mycophenolate mofetil; MP: mesangial proliferation.
Group A: 17 patients (four female) with a mean age of 7.7 years (r = 5.1–11.9). The time elapsed from the start of steroid treatment to the start of anti-proteinuric treatment was 27.5 months (SD 3.1).
The mean final dose of enalapril was 0.4 mg/kg/day (SD 0.2) and that of losartan was 1.2 mg/kg/day (SD 0.4).
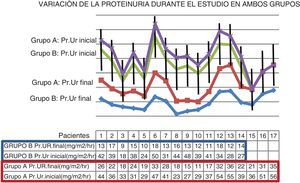
The average initial proteinuria of the 17 patients was 39 mg/m2/h and the mean proteinuria at the end of the study was 24 mg/m2/h, p < 0.01 (Fig. 1). At the end of the 24 months of treatment, none of the patients had reduced the proteinuria to physiological values (Fig. 2).
Change in proteinuria (Pr.Ur) during the study in both groups. In each group the drop in proteinuria was significant, group A: p < 0.01, group B: p < 0.001; however, on comparing the drops in proteinuria in the two groups, the drop in proteinuria was significantly greater in group B (t test: 11.5, p < 0.001).
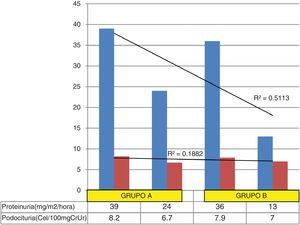
Change in proteinuria and podocyturia in both groups. The coefficient of determination shows that a greater percentage of the change in proteinuria (51%) from the start to the end of the study is not proportionally related to the change in podocyturia (18.8%); this lack of association between the two changes is explained because a very small change in podocyturia values (especially in group B) leads to a significant reduction in proteinuria.
The average podocyturia of the 17 patients at the start of treatment was 8.2 cells/100 mg creatininuria and the mean end podocyturia was 6.7 cells/100 mg creatininuria, p = 0.07.
The inverse correlation coefficient between change in proteinuria and podocyturia was −0.40 at the start of treatment and −0.51 at the end of treatment.
Mean serum creatinine was 0.62 mg/dl (SD 0.2) and GFR was 102 ml/min/1.73 m2 (SD 9).
Mean total serum protein was 4.1 g/dl (SD 1.3) and mean albuminaemia was 2.2 g/dl (SD 0.5), while the mean serum sodio level value was 143 mEq/l (SD 8.3), serum potassium was 4.4 mEq/l (SD 0.7) and urinary sodium was 8 mEq/l.
Finally, mean blood pressure was 66 mmHg (SD 5.5).
Group B: there were 14 patients (5 female) with a mean age of 8.2 years (r = 5.3-11.3). The time elapsed from the start of steroid treatment to the start of anti-proteinuric treatment was 25.8 months (SD 2.8).
The mean final dose of enalapril was 0.4 mg/kg/day (SD 0.2), that of losartan was 1.2 mg/kg/day (SD 0.4) and that of amiloride was 0.3 mg/kg/day (SD 0.05).
The relative risk was 0.33 (95% CI 0.14−0.29), with an absolute risk reduction of 43% (95% CI 0.22−0.83) and a number needed to treat of 2.5 patients.
The average proteinuria of the 14 patients before starting treatment was 36 mg/m2/h and the average proteinuria at the end of the study was 13 mg/m2/h, p < 0.001 (Fig. 1). At the end of the 24 months of treatment, no patient’s proteinuria had reduced to physiological values.
The average podocyturia of the 14 patients at the start of treatment was 7.9 cells/100 mg creatininuria and the mean end podocyturia was 7.0 cells/100 mg creatininuria, p = 0.2.
The inverse correlation coefficient between change in proteinuria and podocyturia was −0.38 at the start of treatment and −0.31 at the end of treatment.
Mean serum creatinine was 0.68 mg/dl (SD 0.1) and average GFR was 96 ml/min/1.73 m2 (SD 7).
Mean total serum protein was 3.9 g/dl (SD 1.1) and mean albuminaemia was 2.3 g/dl (SD 0.8). The mean serum sodium concentration was 136 mEq/l (SD 2.3), serum potassium was 4.0 mEq/l (SD 0.3) and the mean urinary sodium value was 22 mEq/l.
Finally, mean blood pressure was 63 mmHg (SD 2.5).
Comparison of proteinuria in both groups revealed that significant differences in proteinuria reduction (paired t test was 11.5, p < 0.001, and the log-rank test was χ2: 4 (p < 0.05) for patients in group B versus patients in group A (Table 2).
Change in proteinuria in patients in group A versus group B.
| Months of proteinuria follow-up | Group A | Group B | Total | Group A | Group B | Total | Group A | Group B | Total |
|---|---|---|---|---|---|---|---|---|---|
| Number of patients at risk of proteinuria | Number of patients at risk of proteinuria | Number of patients with observed proteinuria | Number of patients with observed proteinuria | Number of patients with expected proteinuria | Number of patients with expected proteinuria | ||||
| 1 | 17 | 14 | 31 | 2 | 2 | 4 | 2.1 | 1.8 | 3.9 |
| 4 | 15 | 12 | 27 | 1 | 2 | 3 | 1.6 | 1.3 | 2.9 |
| 7 | 14 | 10 | 24 | 2 | 2 | 4 | 2.3 | 1.6 | 3.9 |
| 10 | 12 | 8 | 20 | 10 | 1 | 11 | 1.2 | 0.8 | 2 |
| 13 | 11 | 7 | 18 | 0 | 2 | 2 | 1.2 | 0.8 | 2 |
| 16 | 11 | 5 | 16 | 0 | 1 | 1 | 0.7 | 0.3 | 1 |
| 24 | 11 | 4 | 15 | 0 | 1 | 1 | 0.7 | 0.3 | 1 |
Log-rank statistic to compare actuarial survival distributions. The log-rank test (χ2: 4, p < 0.05) showed significant differences in remissions for patients in group B versus patients in group A. The extension by one decimal place is explained by the very small difference in the totals.
The two factors associated with increased proteinuria in the Cox proportional hazards model were podocyturia (OR 14, 95% CI 1.1–20) and time elapsed from SRNS diagnosis to the start of anti-proteinuric treatment (OR 18, 95% CI 1.1–39); we did not find significant risk associated with either age (OR 1.1, 95% CI 0.15–12) or GFR (OR 0.7, 95% CI 0.5–10).
DiscussionWe implemented two distinct non-immunological treatment regimens to reduce proteinuria in paediatric patients with a history of primary SRNS, finding a reduction in proteinuria in both groups, although it was more significant in the group treated with enalapril, losartan and amiloride, without an associated change in the amount urine podocyte.
Although for operational reasons it was not possible to determine the genetic lineage of the nephrotic syndromes included, we do not believe that the lack of this diagnostic tool would be decisive in changing the therapeutic response implemented in this protocol.
To justify the use of these anti-proteinuric therapeutic regimens, it is necessary a brief review of the pathophysiology of glomerular injury resulting from foot process effacement with podocyte detachment, and the development of pathological albuminuria.5–7 The initial events justifies the quantitative measurement of podocyturia as an adequate biomarker to assess glomerular involvement, from the consequent appearance of proteinuria in the mesangium and the onset of cell proliferation with migration and activation of various chemotactic factors; this sequence favours the increasing expansion of the mesangium with gradual extrinsic compression of the glomerular tuft.8–10
The grounds for combined treatment with enalapril and losartan are based on haemodynamic action:, enalapril inhibits angiotensin-2 synthesis and raises bradykinin and prostaglandin levels, causing increased renal blood flow, with a consequent reduction in protein concentrations in the glomerular capillary; on the other hand, losartan blocks the action of angiotensin-2 on the AT1 receptor.11,12
The reduction in proteinuria in the group B patients was significantly greater than in group A, without an associated change in podocyturia. It is possible that the benefitial effect of the addition of amiloride, a sodium channel blocker diuretic that acts mainly on the distal renal tubules13, is explained by its capacity to inhibit integrin ß-3 activation and urokinase receptor expression in its soluble form.14 This interaction would cause the effacement (retraction) of the foot processes15,16 and podocyte detachment; both events are early lesions in the progression towards focal segmental glomerulosclerosis.17–21 In support of this hypothesis, Trimarchi et al. report that patients with untreated Fabry disease present high podocyturia with low proteinuria22 and, in keeping with this report (although it relates to a different disease), we saw a greater reduction in proteinuria with a lower quantitative concentration of podocytes in urine in group B patients compared to those in group A. These variations between the two groups might confirm the existence of an established pattern between time of evolution of the disease and progressive risk of irreversible kidney injury23 and, judging from the outcomes in our population, the use of amiloride would contribute to reducing podocyturia and consequent proteinuria.
The unblinded nature of the study, our inability to measure some of the mediators involved in the pathophysiology of proteinuria such as plasmin, plasminogen and urokinase receptor23,24, together with the operational impossibility of performing a genetic study in disease of this type, are weaknesses of the work. However, its originality resides in the use of amiloride to control two such significant variables as podocyturia and proteinuria in children; there are few reports on this subject25 and we believe that this is the first study in paediatric patients with SRNS.
Conclusions- 1.
Amiloride lowers proteinuria without significant modification of podocyturia, and without any observed adverse effects associated with its use.
- 2.
A longer time elapsed since the onset of kidney disease, accompanied by a reduction in podocyturia values, favoured an increase in proteinuria.
M. Liern participated in the original idea, the preparation of the protocol, the preparation of the article and the statistics. A. Collazo participated in data collection and the preparation of the article. G. Vallejo participated in the preparation of the article and coordination. E. Zotta participated in data collection and the preparation of the article.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Liern M, Collazo A, Vallejo G, Zotta E. Acción antiproteinúrica del amiloride en el paciente pediátrico con síndrome nefrótico corticorresistente. Nefrologia. 2021;41:304–310.