The evaluation of the measured Glomerular Filtration Rate (mGFR) or estimated Glomerular Filtration Rate (eGFR) is key in the proper assessment of the renal function of potential kidney donors. We aim to study the correlation between glomerular filtration rate estimation equations and the measured methods for determining renal function.
Material and methodsWe analyzed the relationship between baseline GFR values measured by Tc-99m-DTPA (diethylene-triamine-pentaacetate) and those estimated by the four-variable Modification of Diet in Renal Disease (MDRD4) and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations in a series of living donors at our institution.
ResultsWe included 64 donors (70.6% females; mean age 48.3±11 years). Baseline creatinine was 0.8±0.1mg/dl and it was 1.1±0.2mg/dl one year after donation. The equations underestimated GFR when measured by Tc99m-DTPA (MDRD4 – 9.4±25ml/min, P<.05, and CKD-EPI – 4.4±21ml/min). The correlation between estimation equations and the measured method was superior for CKD-EPI (r=.41; P<.004) than for MDRD4 (r=.27; P<.05). eGFR decreased to 59.6±11 (MDRD4) and 66.2±14ml/min (CKD-EPI) one year after donation. This means a mean eGFR reduction of 28.2±16.7ml/min (MDRD4) and 27.31±14.4ml/min (CKD-EPI) at one year.
ConclusionsIn our experience, CKD-EPI is the equation that better correlates with mGFR-Tc99m-DTPA when assessing renal function for donor screening purposes.
El estudio del filtrado glomerular medido (FGm) o del estimado (FGe) es el eje de la evaluación adecuada de la función renal en la valoración de un potencial donante vivo renal. Nos planteamos estudiar la correlación entre las fórmulas de estimación del FG y los métodos de medición para determinar la función renal.
Material y métodosAnalizamos la relación entre los valores basales de FGm con Tc-99m-DTPA (dietilenolene-triamino-pentaacetato) y aquellos estimados mediante las fórmulas Modification Diet Renal Disease de 4 variables (MDRD4) y Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) en una serie de donantes vivos de nuestra institución.
ResultadosIncluimos a 64 donantes (70,6% mujeres; con una edad media de 48,3±11 años). La creatinina basal fue 0,8±0,1 y 1,1±0,2mg/dl un año posdonación. Las ecuaciones infraestiman el FG medido por Tc99m-DTPA (MDRD4 −9,4±25ml/min y p<0,05; CKD-EPI −4,4±21ml/min). La correlación entre las fórmulas estimativas y el método medido fue superior para CKD-EPI (r=0,41; p=0,004) que para MDRD4 (r=0,27; p=0,05). El FGe se redujo a 59,6±11 (MDRD4) y a 66,2±14ml/min (CKD-EPI) al año posdonación. Esto supone una reducción media del FGe de 28,2±16,7ml/min (MDRD4) y de 27,31±14,4ml/min (CKD-EPI) al año.
ConclusiónEn nuestra experiencia, CKD-EPI es la fórmula que mejor se correlaciona con el FGm-Tc99m-DTPA en la evaluación de la función renal para el cribado de donantes.
Since 1954, the year the first successful living-donor kidney transplant was performed,1 this technique has expanded greatly and proven to be not only an important source of organs, but also the best replacement therapy for patients with advanced chronic kidney disease.2 In Spain, this modality has grown significantly in the last decade, and has reached a rate of 9 interventions per million inhabitants per year.3
Various single-center and multi-center studies have published up to 2010 data from long-term follow-up of kidney donors and they have not found a greater risk of chronic kidney disease or death compared to the general population.4–9 However, more recent studies that have included a more appropriate paired control group have indicated that there could be an increased long-term risk. Unfortunately, these studies have some significant biases rendering their results difficult to interpret and extrapolate to regular clinical practice.10,11
There is no controversy with respect to the need to study the potential kidney donor in depth, in order to prevent a negative impact on his or her health and to achieve the best outcome in the recipient.12–14 A key element within the study of potential donors is evaluation of kidney function. Measurement of glomerular filtration rate (mGFR) using non-isotope external markers (inulin, iohexol or iothalamate) is considered the gold standard test for determining kidney function.12 However, these techniques require a long, complex procedure. This limits their use in regular clinical practice. Some centers use less costly isotope techniques (diethylenetriamine acid marked with technetium [Tc99m-DTPA], EDTA-Cr51, 125iothalamate),15 and others base evaluation of kidney function exclusively on calculation of estimated glomerular filtration rate (eGFR) using formulas centered on serum creatinine. The most commonly used formulas are the 4-variable Modification of Diet in Renal Disease (MDRD-4) formula and the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) formula. The main limitation of the MDRD-4 formula is that it shows a low correlation with mGFR if values exceed 60ml/min.16 The CKD-EPI formula was developed from a cohort that, unlike MDRD-4, included individuals with normal kidney function in addition to patients with limited kidney function. This provides a better correlation with mGFR in healthy subjects.17 Despite its validation in the general population, there are not enough studies confirming its precision for evaluating kidney function in potential living donors.13–15
The objective of our study was to compare the mGFR method using an isotope renogram with Tc99m-DTPA and the estimation formulas based on serum creatinine in order to evaluate these formulas’ validity in a potential kidney donor study, as well as subsequent recipient and donor follow-up.
MethodsA retrospective study was performed in which 64 healthy individuals who consecutively underwent a nephrectomy for kidney donation at Hospital del Mar in Barcelona were evaluated between January 2001 and March 2015. mGFR was performed using a renogram with Tc99m-DTPA 2–6 months before nephrectomy, and eGFR was performed using plasma creatinine-based formulas according to MDRD-4 and CKD-EPI as part of the living donor study protocol. The creatinine value closest to the donation date was taken as the reference.
All procedures were performed in accordance with the Declaration of Helsinki, and all donors signed an informed consent form that included the procedures featured in this study.
Measurement of kidney functionAn isotope renogram was performed, with administration as an intravenous bolus of 5–10mCi (370Mbq) of Tc99m-DTPA, a radioactive isotope whose activity can be detected in the human body through special cameras. Most Tc99 elimination is by glomerular filtration, with no tubular resorption or secretion and no adhesion to plasma proteins. Every 30–60s, computed images are taken in order to evaluate renal artery perfusion, transit through the renal parenchyma and excretion of the radiotracer through the renal pelvis, ureters and bladder.
Estimation of kidney function: creatinine-based equationseGFR was obtained by means of determination of creatinine performed with traceability to the reference method for isotope dilution mass spectrometry (IDMS) in blood samples using the following formulas:
- (1)
4-variable MDRD (IDMS) (MDRD-4)=175×(creatinine)−1.154×(age)−0.203×0.742 (if a woman)×1.210 (if black race).
- (2)
CKD-EPI calculated by gender and stratified by creatinine.
Females
Creatinine≤0.7mg/dl: eGFR=144×(creatinine/0.7)−0.329×(0.993)age×1.159 (if black race).
Creatinine>0.7mg/d: eGFR=144×(creatinine/0.7)−1.209×(0.993)age×1.159 (if black race).
Males
Creatinine≤0.9mg/dl: eGFR=141×(creatinine/0.9)−0.411×(0.993)age×1.159 (if black race).
Creatinine>0.9mg/dl: eGFR=141×(creatinine/0.9)−1.209×(0.993)age×1.159 (if black race).
Statistical analysisQuantitative variables with a normal distribution were expressed as mean and standard deviation (SD), and all other variables were expressed as median and interquartile range. All continuous variables had a normal distribution confirmed by the Kolmogorov–Smirnov test. We used the intraclass correlation coefficient (ICC) to determine whether differences in GFR measurement using different measuring instruments were due to the subject who measured them and not to the method itself. This coefficient estimates the average of the correlations between all possible ordinations of the pairs of observation available, and therefore circumvents the problem of dependence of the order of Pearson's correlation coefficient (r), which only takes linear correlation into account. All statistical analyses were performed with the software program SPSS version 20.0 (SPSS Inc., Chicago, IL, United States). A p value<0.05 was considered to be statistically significant.
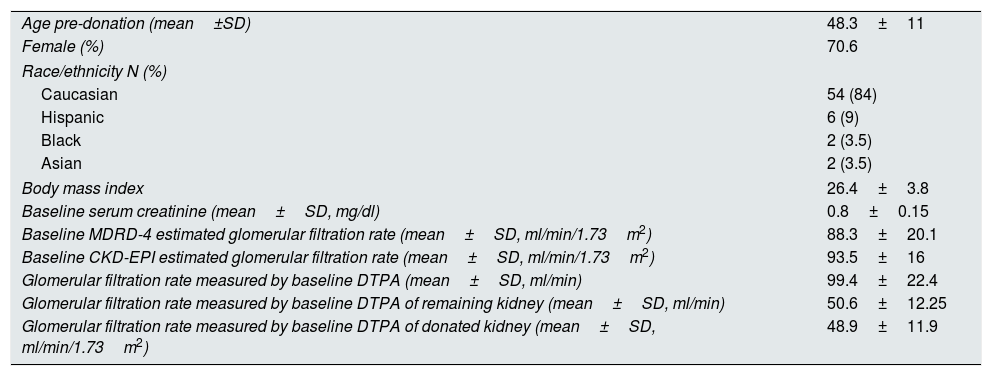
ResultsA total of 64 consecutive kidney donors were enrolled between 2001 and March 2015 (Table 1). Their mean age was 48±11 years, their mean body mass index was 26.4±3.8kg/m2, 70.6% were females and 84% were Caucasian. Four donors (6.6%) had well-controlled hypertension with drugs treatment at the time of donation. Two donors (3.3%) had a history of diabetes mellitus with normal baseline blood sugar and no need for drugs at the time of donation. None of them had a history of cardiovascular or kidney disease. Before kidney donation, mean serum creatinine was 0.8±0.15mg/dl. Baseline GFR measured using Tc99-DTPA was 99.4±22.4ml/min, and baseline eGFR measured using MDRD-4 and CKD-EPI was 88.3±19.3 and 93.5±15.9ml/min, respectively. mGFR by DTPA for the donated kidney was 48.9±11.9ml/min, whereas that for the remnant kidney was 50.6±12.2ml/min. A year after donation, none of the donors had died or required renal replacement therapy.
Baseline characteristics of the 64 kidney donors enrolled in the study.
| Age pre-donation (mean±SD) | 48.3±11 |
| Female (%) | 70.6 |
| Race/ethnicity N (%) | |
| Caucasian | 54 (84) |
| Hispanic | 6 (9) |
| Black | 2 (3.5) |
| Asian | 2 (3.5) |
| Body mass index | 26.4±3.8 |
| Baseline serum creatinine (mean±SD, mg/dl) | 0.8±0.15 |
| Baseline MDRD-4 estimated glomerular filtration rate (mean±SD, ml/min/1.73m2) | 88.3±20.1 |
| Baseline CKD-EPI estimated glomerular filtration rate (mean±SD, ml/min/1.73m2) | 93.5±16 |
| Glomerular filtration rate measured by baseline DTPA (mean±SD, ml/min) | 99.4±22.4 |
| Glomerular filtration rate measured by baseline DTPA of remaining kidney (mean±SD, ml/min) | 50.6±12.25 |
| Glomerular filtration rate measured by baseline DTPA of donated kidney (mean±SD, ml/min/1.73m2) | 48.9±11.9 |
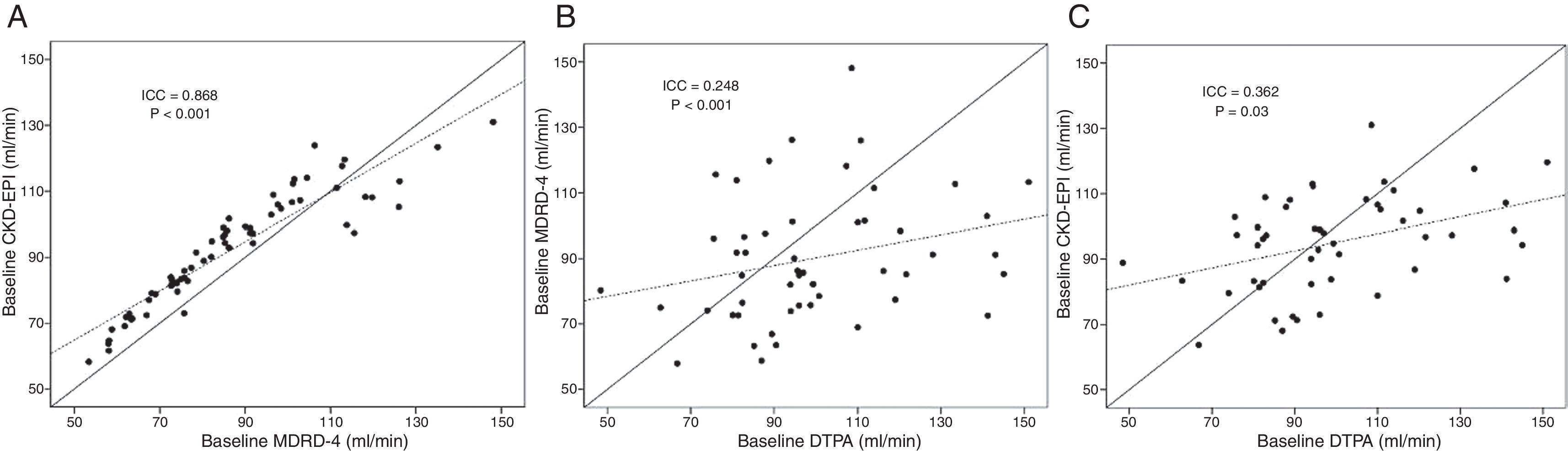
Both MDRD-4 and CKD-EPI show a significant linear correlation with the mGFR method. This correlation was more significant with CKD-EPI (r=0.41; p=0.004) than with MDRD-4 (r=0.27; p=0.05) (Fig. 1). If the same analysis is performed while distinguishing by gender, similar results are maintained for CKD-EPI (women r=0.36 and p=0.03; males r=0.57 and p=0.02); however, the significance of the correlation between mGFR and MDRD-4 is lost.
Correlation between the various glomerular filtration rate estimation and measurement methods. (A) Correlation between the estimation formulas MDRD-4 and CKD-EPI. (B) Correlation between GFR estimated by MDRD-4 and that measured with Tc99-DTPA. (C) Correlation between GFR estimated by CKD-EPI and that measured with Tc99-DTPA. ICC: intraclass correlation coefficient; GFR: glomerular filtration rate.
The ICC between baseline MDRD-4 and baseline CKD-EPI was 0.86 (p<0.001). This indicated a good correlation between the two formulas (Fig. 1). However, if measurement by DTPA is correlated with the estimation formulas, the ICC showed a limited correlation for both CKD-EPI and MDRD-4. This correlation was somewhat better for the first formula (ICC=0.362; p=0.025) than for the second formula (ICC=0.248; p<0.001). An analysis by gender confirmed that CKD-EPI increased its power of correlation with mGFR for both sexes (females ICC=0.49 and p=0.02; males ICC=0.65 and p=0.03) and MDRD-4 maintained a limited correlation with DTPA.
Both formulas underestimated GFR compared to mGFR. The difference between mGFR and eGFR was statistically significant with MDRD-4 (9.4±25ml/min lower than that measured by Tc99-DTPA, p<0.05), but not with CKD-EPI (4.4±21ml/min lower than mGFR, p=0.145). In addition, eGFR by MDRD-4 was 5ml/min lower than that estimated by CKD-EPI (p<0.001).
The mGFR in the two kidneys of the donor, show a non-significant difference of 1.7±8ml/min (p=0.145) was observed between the transplanted kidney and the remaining kidney: mGFR was always greater for the remaining kidney.
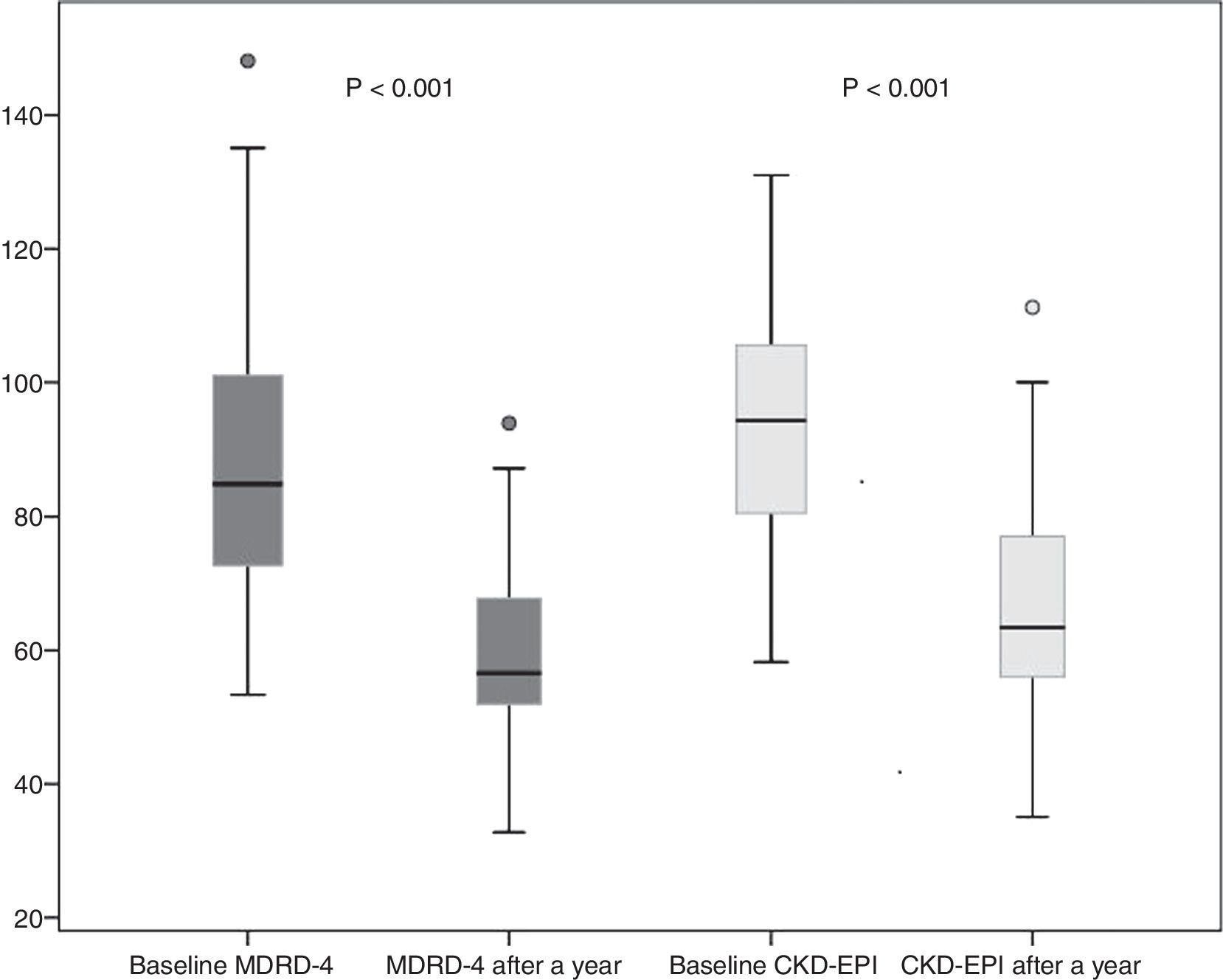
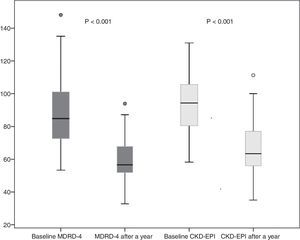
Donor kidney function outcomes after a yearSixty donors completed 12 months follow-up, and 4 were lost to follow-up. A year after donation, donor creatinine was 1.1±0.2mg/dl. eGFR decreased to 59.6±11 and 66.2±14ml/min by MDRD-4 and CKD-EPI, respectively. This represents a mean decrease in eGFR after one year (as compared to baseline eGFR) of 28.2±16.7ml/min by MDRD-4 and 27.31±14.4ml/min by CKD-EPI (Fig. 2).
DiscussionIn this study, we have analyzed a cohort of living kidney donors in whom kidney function was evaluated prior to nephrectomy with measurement methods and estimation formulas based on serum creatinine. Our results indicate that the degree of correlation between estimation formulas and mGFR through DTPA is limited: both formulas underestimate GFR compared to the value obtained by DTPA. However, this effect seems to be less marked with the CKD-EPI formula.
Prior studies that have compared the use of MDRD-4 and CKD-EPI to evaluate kidney function in a potential donor with one of the measurement methods available have yielded disparate results. Macías et al.18 compared mGFR to EDTA-Cr51 and the estimation formulas, and found better correlation in GFR values provided by MDRD-4 and MDRD-6 than by CKD-EPI. Therefore, the authors recommended MDRD as a tool for screening potential kidney donors. In closer alignment with our results, Lujan et al.19 compared GFR measured with iothalamate and estimated with MDRD-4 and CKD-EPI in 85 donors. They found greater precision and less bias with CKD-EPI than with MDRD-4. Other studies have yielded suitable linear correlation coefficients between estimation formulas (with no differences between them) and mGFR with iohexol.19,20
Proper evaluation of the kidney function in a potential donor is critical, given its decisive impact on the future function of the graft and of the kidney remaining in the donor. Following nephrectomy, the donor loses 50% of his or her GFR. Compensation on the part of the remaining kidney reaches 70% of initial function. The factor that most strongly influences the ultimate function attained is GFR prior to donation; however, age, sex, race and body size also play a role.15,20 Our study found that donor kidney function compensation a year after donation compared to baseline was 67.8% if measured using MDRD-4 and 70.8% if measured using CKD-EPI.
Despite its importance, there is no general consensus on the threshold of kidney function that should allow potential donors to undergo nephrectomy. All recommendations on evaluation of kidney function prior to nephrectomy are based on expert opinion. For the moment, there are no randomized studies with long-term follow-up that address the minimum GFR threshold for kidney donation or the best method for determining it.21,22
There is also no agreement with respect to the best method to determine the kidney function of a potential donor. The use of a measurement method is considered to be advisable since, as several prior studies have indicated, approximation (correlation and percentage of error) of GFR estimated by formulas or creatinine clearance to real measured GFR is limited.18–20,23 Thus, given the inaccuracy of estimation methods, some guidelines, including the Spanish guidelines, recommend measuring it directly with any method, mainly in cases of kidney function close to the threshold proposed for donation.24,25 The most recent European guidelines assume a margin of error for estimation formulas, but do not consider it to be of sufficient clinical significance as to recommend strict use of mGFR: they defend estimation formulas as a main method, thereby relegating measurement methods to uncertain cases.22 In a multitude of transplant programs, the GFR of the potential donor is only estimated; it is not measured. Therefore, it is very important to search for a formula that more closely corresponds to actual GFR and to address the matter of the actual utility of the measurement methods used.
The main limitation of our study was the sample size analyzed. This made it difficult to extrapolate the data obtained to the general donor population. Moreover, our study did not include measurement of donor GFR after a year. Therefore, the precision analysis of the formulas was limited to the baseline study.
In conclusion, the CKD-EPI formula for estimation of GFR is the one that correlates best with FGm-Tc99m-DTPA in baseline evaluation of the kidney donor, and the use of this formula seems to be more appropriate than the use of the MDRD-4 formula. Both formulas underestimate mGFR. Therefore, it would seem reasonable to think that, above a particular eGFR, kidney donation would be safe, given that mGFR will probably be higher. However, the best evaluation of kidney function prior to donation remains a combination of both formulas, particularly in cases where kidney function is close to the threshold considered to be advisable for donation.
FundingThis study was made possible in part by the funding of the projects FIS-FEDER PI13/00598, FIS-FEDER PI16/00617 and RETIC FEDER RD16/0009/0013 (RedinRen).
Conflicts of interestThe authors declare that they have no conflicts of interest related to the content of this article.
The authors would like to thank Sara Álvarez, Montserrat Folgueiras, Anna Faura, María Vera, Raquel Martín, Ernestina Junyent and the entire nephrology nursing team at Hospital del Mar for their invaluable collaboration in this project.
Please cite this article as: Burballa C, Crespo M, Redondo-Pachón D, Pérez-Sáez MJ, Mir M, Arias-Cabrales C, et al. MDRD o CKD-EPI en la estimación del filtrado glomerular del donante renal vivo. Nefrologia. 2018;38:207–212.