Type iii extracapillary glomerulonephritis (PEGN) is a common cause of rapidly progressive glomerulonephritis and it is usually associated with circulating anti-neutrophil cytoplasmic antibodies (ANCAs). Recent evidence points to complement activation as an important factor in the pathogenesis of PEGN.
The aim of the present study was to assess the value of C3 deposits in the prognosis of PEGN.
MethodsAll patients diagnosed of PEGN from 1995 to 2015 (n=72) were included in this study. Progression of renal disease in patients with positive staining for C3 by immunofluorescence was compared with those with negative staining. Mean follow up was 73 months. Progression to end-stage renal disease in relation to clinical and histological variables was analyzed.
ResultsPositive staining for C3 was observed in 22 out of the 72 patients (30.5%).
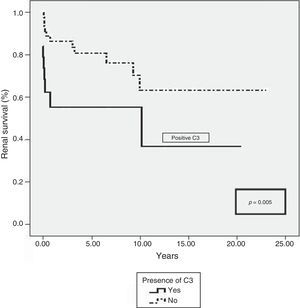
At the time of diagnosis, patients with C3 deposits had higher serum creatinine concentration than those without C3 staining (5.00 vs. 3.85mg/dl, p=0.050). Renal survival at 10 years was 36.9% in patients with positive C3 staining vs. 64.4% in patients with negative staining (p=0.005). Mortality at 10 years was higher in patients with C3 deposits than in patients without deposits (77 vs. 49.3%).
ConclusionsThus, our study shows that PEGN with deposits of C3 is associated with worse renal prognosis and greater mortality. These results would support the hypothesis that activation of the alternative pathway complement may play an important role in the generation of renal injury associated with PEGN.
La glomerulonefritis extracapilar (GNEC) pauciinmune o de tipo iii es una de las causas más comunes de glomerulonefritis rápidamente progresiva y suele estar asociada con la presencia de anticuerpos antineutrófilos citoplasmáticos (ANCA). Están reportándose evidencias sobre la importancia de la activación del complemento en la patogénesis de la GNEC.
El objetivo de nuestro estudio fue evaluar el papel pronóstico del depósito de C3 en las GNEC de tipo iii.
MétodosSe estudió a pacientes diagnosticados de GNEC de tipo iii entre 1995 y 2015 (n=72). Comparamos a pacientes con tinción positiva para C3 en el estudio de inmunofluorescencia con aquellos con tinción negativa. Se analizaron variables clínicas e histológicas y se relacionaron con progresión a enfermedad renal terminal.
ResultadosSe encontró tinción positiva para C3 en 22 pacientes de un total de 72 (30,5%).
Basalmente los pacientes con depósitos de C3 tenían peor función renal que aquellos sin depósitos (creatinina sérica 5 vs. 3,85mg/dl; p=0,050). La supervivencia renal a los 10 años fue del 36,9% en los pacientes con tinción positiva para C3 frente al 64,4% en los pacientes con tinción negativa (p=0,005). La supervivencia a los 10 años fue peor en los pacientes con depósitos de C3 (77 vs. 49,3%).
ConclusionesNuestro estudio revela que la presencia de depósito de C3 en la GNEC de tipo iii se asocia a un peor pronóstico renal y de la supervivencia del paciente. Estos resultados son compatibles con la hipótesis de que la activación de la vía alternativa del complemento contribuye al daño renal asociado a la GNEC de tipo iii.
Extracapillary glomerulonephritis (ECGN) type III is a severe glomerular disease that is associated with a progressive deterioration of renal function. The term extracapillary glomerulonephritis is generally used in glomerulonephritis with crescents in more than 50% of the glomeruli. It is not a specific disease but rather a manifestation of severe glomerular damage caused by many etiological factors.1,2
Classically, it has been associated with the presence of antineutrophil cytoplasmic antibodies (ANCA). The ANCA are mainly directed to 2 antigens, myeloperoxidase and proteinase.3
Some aspects of the pathogenesis of this disease are not clear. In the past, it was assumed that the role of complement was minimal.4 Presently, with the recent advances, it is postulated that the activation of the alternative pathway of the complement could contribute to the pathogenesis of this disease. Experimental models have demonstrated the importance of alternative pathway of complement activation in this entity.5
Only few studies have analyzed the presence of complement in kidney samples. Chen et al.6 observed C3c deposits in 33% of patients with type III ECGN. The presence of C3c deposits was associated with more severe renal insufficiency and increased proteinuria at the onset of type III ECGN. Xing et al.7 detected the presence of C3d in glomeruli and small blood vessels in renal samples from patients with type III ECGN. Hilrost et al.8 found complement factors in renal biopsies of patients with type III ECGN and the presence of C3d and properdin was associated with a greater proportion of crescents and greater proteinuria. Recently, Villacorta et al.9 showed demonstrated that the presence of C3d is an independent risk factor for survival in type III ECGN.
The aim of our study was to evaluate the short and long term prognostic value of C3 deposits in patients with type III ECGN.
MethodsPatientsThis is a retrospective observational study including 72 patients with the histological diagnosis of type III ECGN during the years 1995–2015 at the Reina Sofía University Hospital (Córdoba). Demographic, clinical and analytical variables were collected at the time of the biopsy and the analytical and clinical course was observed during the years of follow-up.
In each patient, the reference point was established at the time of biopsy. The follow-up time was established as the interval between the renal biopsy and the last follow-up visit, death or the need for renal replacement therapy (dialysis or renal transplant). The main objective of the study was to analyze whether in type III ECGN the prognosis is modified by the presence of C3 deposits detected by immunofluorescence.
Histological characteristicsRapidly progressive type III or pauciimmune glomerulonephritis was defined histologically as the presence of extracapillar proliferation in the absence of significant glomerular immune deposits.
The renal biopsy samples were analyzed by optical microscopy and immunofluorescence. For optical microscopy the samples were stained with hematoxylin-eosin, Masson's trichrome and methenamine silver. The biopsies were analyzed by the expert pathologist in the reference center. The degree of tubular atrophy, interstitial fibrosis, glomerulosclerosis and intra- and extracapillary proliferation were analyzed in all samples. The fragment to be use for immunofluorescence was frozen in fresh to make 4μ cuts with the cryostat; it was fixed in acetone before staining and it was processed with immunoreactants for IgG, IgA, IgM, complement (C3 and C1q), fibrinogen and light chains (kappa/lambda). The visualization was done in the dark field microscope with ultraviolet light.
A sample was positive for C3 if the presence of C3 was 1+ (mild), 2+ (moderate) or 3+ (severe) and C3 negative or absent if there was no evidence of C3 deposits by immunofluorescence. According to the histological classification described by Berden,10 there were 4 classes: (1) the sclerotic class if there were at least 50% of the sclerosed glomeruli; (2) the crescentic class if there was equal or more than 50% of crescents; (3) the focal class, if at least 50% of the glomeruli were normal and (4) the mixed class if there was a combination of the crescentic, focal and sclerotic class in less than 50% of the glomeruli. Tubular atrophy and interstitial fibrosis were defined as absent (0%), mild (<25%), moderate (25–50%) or severe (>50%).
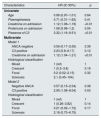
Statistical analysisContinuous variables were compared by Student's t-test. The differences between the proportions of the two groups were evaluated by chi square test. The time of follow-up of each patient was the difference between the initial point of reference and the arrival to RRT or last visit. A Cox regression model was used to estimate the adjusted relative risk of each parameter with respect to renal survival. Variables previously found to affect renal survival were included in the model. The variables with non-statistically significant effect were eliminated according to the model of backward step. In the multivariate analysis, 2 models were evaluated, one that included the histological classification, the creatinine at admission, the presence of C3 and the presence of ANCA. In a second model, creatinine on admission was excluded, as it was significantly higher in C3 positive patients (5mg/dl) than in negative C3 patients (3.85mg/dl, p=0.050). Long-rank model was used to compare survival. The level of statistical significance was set at 0.05. The calculations were made with SSPS (version 15.0).
ResultsDemographic and analytical characteristics of the 72 patients are shown in Table 1.
Demographic and analytical characteristics.
| C3 positive (n=22) | C3 negative (n=50) | Total N=72 | p | |
|---|---|---|---|---|
| Age (years) | 64 (46–78) | 61.50 (55–70.55) | 63.5 (54.25–72) | 0.63 |
| Gender, males n (%) | 15 (68.2) | 31 (63.3) | 46 (64.8) | 0.79 |
| HTN n (%) | 10 (45.5) | 23 (46.9) | 33 (46.5) | 0.55 |
| Diabetes n (%) | 4 (18.2) | 6 (12.2) | 10 (14.1) | 0.48 |
| Low C3 levels n (<75) (%) | 2 (9.5) | 2 (4.2) | 4 (5.8) | 0.58 |
| P_ANCA n (%) | 10 (45.5) | 30 (61.2) | 40 (56.3) | 0.3 |
| C_ANCA n (%) | 3 (13.6) | 15 (30.6) | 18 (35.4) | 0.15 |
| ANCA negative n (%) | 10 (45.5) | 8 (16.3) | 18 (25.4) | 0.02 |
| Immunosuppression (CTC, CFF, AZA, rituximab) n (%) | 18 (94.7) | 46 (95.8) | 64 (95.5) | 0.63 |
| Plasmapheresis n (%) | 6 (31.6) | 16(33.3) | 22 (32.8) | 0.65 |
| Follow-up (months) | 73.50 | 70 | 73 | 0.65 |
| Creatinine at admission (mg/dl) | 5 (4.6–8.1) | 3.85 (2.52–5.22) | 4.50 (3.42–6.07) | <0.05 |
| Proteinuria on admission (g) | 3 (1.7–6) | 1.45 (0.75–2.95) | 1.9 (0.95–3.5) | 0.20 |
| Creatinine at follow up (mg/dl) | 3.03 | 1.69 | 1.70 | <0.01 |
| HD on admission | 10 (52.6) | 18 (36.7) | 28 (41.2) | 0.27 |
| ESRD n (%) | 10 (54.5) | 36 (26.5) | 46 (35.2) | 0.03 |
| Deaths n (%) | 11 (50) | 8 (16.3) | 19 (26.8) | <0.01 |
AZA: azathioprine; C-ANCA: antineutrophil cytoplasmic antibodies antiproteinase; CFF: cyclophosphamide; CTC: corticosteroids; HD on admission: hemodialysis on admission; HTN: hypertension; P-ANCA: antineutrophil cytoplasmic antibodies antimyeloperoxidase; ESRD: end stage renal disease; M: males.
The majority of patients were men (64.8%), with mean age of 63.5 years. The creatinine on admission was 4.50mg/dl and the proteinuria was 1.9g/day. A 41.2% of patients required dialysis on admission. ANCA was positive in 85% of patients, 56.3% for P-ANCA and 35.4% for C-ANCA. All patients except one received immunosuppression treatment that in most cases included steroids and cyclophosphamide; other cases were treated with steroids plus azathioprine or rituximab.
In 22 patients (30.5%) presented deposits of C3 by IF and in 50 patients (69.5%) there was no evidence of C3 deposits. Comparison of patients with positive and negative C3 reveal no significant differences in terms of the number of sclerosed glomeruli, crescents, degree of tubular atrophy, interstitial fibrosis or histological classification (Table 2). In only 2 patients with positive C3 (9.09%) and in one patient (2%) with negative C3 there was deposit of C1q (+). None of the patients had immunoglobulin deposits greater than 1+. The average number of glomeruli obtained in the renal samples was 14.5; 22% of the glomeruli had sclerosis. The average percentage of crescents in each renal sample was 51%. Tubular atrophy was mild in 50% and moderate in 30% of cases. Interstitial fibrosis was mild in 51.4% and moderate in 35.7%. According to the Berden classification, 49.3% were classified as crescentic, 21.1% as focal, 14.1% as sclerosis and 15.5% as mixed; histological classification was not significantly different i patients with and without C3 staining.
Histological characteristics.
| C3 positive (n=22) | C3 negative (n=50) | Total | p | |
|---|---|---|---|---|
| Number of glomeruli | 12.73±5.88 | 14.94±9 | 14.51±8.4 | 0.22 |
| Number of glomeruli with sclerosis | 2.41±2.87 | 3.39±3.5 | 3.2±3.55 | 0.22 |
| Crescents | 7.41±4.36 | 7.22±6.7 | 7.42±6.1 | 0.89 |
| Tubular atrophy n (%) | ||||
| Absent | 2 (9.1) | 7 (14.6) | 9 (12.9) | 0.8 |
| Mild | 7 (59.1) | 26 (54.2) | 39 (55.7) | |
| Moderate | 13 (31.8) | 14 (29.2) | 21 (30) | |
| Severe | 0 (0) | 1 (2.1) | 1 (1.4) | |
| Interstitial fibrosis n (%) | ||||
| Absent | 1 (4.5) | 6 (12.5) | 7 (10) | 0.47 |
| Mild | 15 (68.2) | 21 (43.8) | 36 (51.4) | |
| Moderate | 6 (27.3) | 19 (39.6) | 25 (35.7) | |
| Severe | 0 (0) | 2 (4.2) | 2 (2.9) | |
| Histológic classification n (%) | ||||
| Crescentic | 15 (68.2) | 20 (40.8) | 35 (49.3) | 0.35 |
| Focal | 1 (4.5) | 14 (28.6) | 15 (21.1) | |
| Sclerótic | 2 (9.1) | 8 (16.3) | 10 (14.1) | |
| Mixed | 4 (18.2) | 7 (14.3) | 11 (15.5) | |
Twenty-two patients (30.5%) were classified as positive C3. The C3 deposits were mild (+) in 17 patients (77.28%) and moderate (++) in 5 patients (22.72%). Four of these 5 patients with C3 (++) were negative for ANCA, 4 were classified histologically as crescentic and 2 of the 5 patients had a paraprotein in blood. Positive C3 patients had negative ANCA in a higher proportion than the C3 negative patients (45.5 vs. 16.3%, p=0.02). As far as the histologic classification there were no significant differences in patients with and without C3 deposits (Table 2).
The creatinine at admission was significantly higher in patients with positive C3 than with negative C3 (5 vs. 3.85mg/dl, p<0.05). During the follow up, patients with positive C3 also presented higher serum creatinine levels than C3 negative patients, (3.03 vs. 1.69mg/dl, p<0.01), this is excluding patients who reached ESRD. Although not statistically significant, a greater proportion of C3 positive patients required renal replacement therapy by means of hemodialysis at admission (52.6 vs. 36.7%, p=0.27).
After a median follow-up of 73 months, 35.2% of the patients reached ESRD and 26.8% died. The renal survival at 10 years according to the histological class was 83% for focal, 70% for mixed, 45% for crescentic, and 0% for sclerosis (p=0.049) (Table 3).
Univariate and multivariate Cox regression analysis. Renal survival is the dependent variable.
| Characteristics | HR (IC 95%) | p |
|---|---|---|
| Univariate | ||
| Age | 0.99 (0.97–1.01) | 0.64 |
| Plasmapheresis | 0.71 (0.31–1.62) | 0.41 |
| Creatinine on admission | 1.12 (1.06–1.19) | <0.01 |
| Proteinuria on admission | 1.08 (0.99–1.2) | 0.04 |
| Presence of C3 | 3.32 (1.16–9.51) | <0.01 |
| Multivariate | ||
| Model 1 | ||
| ANCA negative | 0.59 (0.17–2.00) | 0.39 |
| C3 positive | 2.23 (0.8–6.17) | 0.12 |
| Creatinine on admission | 1.12 (1.04–1.21) | <0.01 |
| Histological classification | ||
| Mixed | 1 (ref) | 1 |
| Crescent | 1 (0.2–3.8) | 0.19 |
| Focal | 0.2 (0.02–2.15) | 0.32 |
| Sclerosis | 2.1 (0.45–104) | |
| Model 2 | ||
| Negative ANCA | 0.57 (0.15–2.04) | 0.38 |
| C3 positive | 2.95 (1.08–8.04) | 0.03 |
| Histological classification | ||
| Mixed | 1 (ref) | 1 |
| Crescent | 1 (0.26–3.82) | 0.14 |
| Focal | 0.21 (0.02–1.72) | 0.17 |
| Sclerosis | 2.18 (0.70–6.75) | |
ANCA: antineutrophil cytoplasmic antibodies.
Patients with positive C3 progressed more frequently to ESRD (54.5% vs. 26.5%, p=0.03). Renal survival was longer in patients with negative C3 at 5 years (88% vs 55% in C3 positive) and at 10 years (63.4% vs 36.9% in C3 positive) (p=0.005) (Fig. 1). Survival was also more favorable in patients with negative C3 at 5 years (92%) and at 10 years (77%) than in positive C3 patients (85 and 49.3%, respectively, p=0.006).
Two Cox regression models were developed. The first included the following variables: histological classification, serum creatinine on admission, presence of C3 and presence of ANCA in renal samples. According to this model, only creatinine at admission turned out to be an independent risk factor for a negative outcome (1.12, 1.04–1.21, p<0.01) (Table 3). Since serum creatinine at admission was significantly higher in positive than negative C3 patients, we developed a second model in which creatinine at admission was excluded. According to this model, only the presence of C3 deposit was found to be an independent risk factor for the development of ESRD (2.95, 1.08–8.04, p=0.02) (Table 3).
DiscussionExtracapillary glomerulonephritis type III is a glomerular disease with a severe negative outcome. In our series, which included all cases consecutively diagnosed during a 20 years period, the 5-year renal survival was 64.8%, a favorable value as compared with other publications (58.7%).11,12
There are factors clearly involved in the in the short-term prognosis of the disease; the histological evaluation helps to anticipate disease progression. Optical microscopy analyzes many parameters that reflect the severity of the tissue lesions such as the percentage of sclerosed glomeruli, percent of normal glomeruli, percent of crescents, degree of fibrosis and tubular atrophy, among others.13–18 Our data are in agreement with published series regarding survival in relation to the histological class: the subclasses with better survival are focal (83% at 10 years) and mixed (70% at 10 years). As expected, the sclerotic subclass is associated to a lowest survival (0% at 10 years), similar to the observations previously described by Berden et al.10
In our study, we analyzed the clinical and histological factors that may be involved in the short and long-term outcome of type III ECGN. Specifically we have evaluated whether the deposition of C3, shown by immunofluorescence, may predict short and long term prognosis of type III ECGN.
This retrospective study shows that among patients with type III ECGN, those with C3 deposits do have a worse short-term prognosis. At the time of biopsy, patients with C3 deposits had serum creatinine levels significantly elevated as compared with patients without C3 deposits (5.00 vs. 3.85mg/dl, p<0.05); furthermore, C3 positive patients required dialysis in a greater proportion (53.6 vs 36.7%, p=0.27) than patients who did not have a C3 deposit. Also important is the fact that the deposit of C3 is associated with worse renal survival (36.9 vs. 64.4% at 10 years in positive vs. negative C3). In the multivariate analysis excluding serum creatinine level at the time of diagnosis, the presence of C3 deposits is an independent predictor of a poor outcome. This has already been shown recently by Villacorta et al.,9 who also demonstrated that in type III GNEC the presence of C3d by immunohistochemistry is an independent risk factor for the development of ESRD.
In the pathogenesis of this disease there are etiological factors that are not fully understood. Recent studies associate the activation of complement with this entity. In the past, it was assumed that the role of the complement was minimal, since renal vasculitis associated with ANCA have been characterized by small glomerular deposit of complement and immunoglobulin factors.4 Neumman et al. demonstrated that the presence of IgG deposits resulted in increased proteinuria and could be associated with a worse prognosis.19
Several studies have shown an important role of complement in vasculitis associated with ANCA20-23. Recent studies indicate that neutrophils are a source of complement factors and that their activation may cause alterations in complement proteins in areas of glomerular injury.24 Xiao et al. point out that stimulation of neutrophils causes the release of factors that activate complement through the alternative pathway, which initiates an inflammatory amplification that mediates glomerular lesions.21–26 Huugen et al.20 and Xiao et al.21 have found that C5 plays a fundamental role in the participation of complement in the vasculitis associated with ANCA. Xiao et al.21 have shown in mouse models that blockade of C5 produces less severe glomerulonephritis. More recently, Hilhorst et al.8 analyzed renal biopsies of patients with the diagnosis of ANCA-associated glomerulonephritis and found positive staining for C3d, C4d and C5b-9 in most of the samples. Also, they have found an association between the presence of C3d and properdin and a greater proteinuria with higher percentage of crescents. Xing et al.7 found C3d in fibrocellular crescents in 7 patients with ANCA-myeloperoxidase.
These data support the hypothesis that activation of the alternative pathway of complement may be involved in the pathogenesis of these glomerulonephritis and that it may condition a worse short- and long-term prognosis. This different activation of the complement may have its origin in predisposing genetic factors, not well identified, or be a consequence of different environmental factors. It has been hypothesized that a greater degree of activation and granulation of neutrophils in ECGN patients with negative ANCA could result in a greater activation of the complement and a more severe injury.27 We need to understand the participation of these factors before making recommendations for clinical management of these patients. Nevertheless, the idea of using monoclonal antibodies against complement suppressants in the future is always open.
It is also important to note that in 5 patients the deposit of C3 was moderate (++). The fact that 4 of them were ANCA negative and that in 2 of them had paraprotein clearly points to the important pathogenic role of the activation of the alternative pathway of complement. Being strict with the definition of C3 glomerulopathy, and according to the latest consensus,28 our C3 positive patients could be considered within the spectrum of C3 glomerulopathy. In this sense, paraproteins have been detected in 31% of patients with C3 glomerulonephritis. It is thought that active paraprotein is an anti-FH antibody that deregulates the alternative pathway of complement.29
Our observations are limited by the retrospective nature of our study. More studies are needed to analyze whether the deposit of C3 is associated with a worse short- and long-term prognosis. However, our study has a long-term clinical and analytical follow-up, all diagnosed by means of a renal biopsy and carefully analyzed by a specialized pathologist.
In conclusion, our study shows that in patients diagnosed with type III ECGN the deposit of C3 is associated with a worse prognosis of renal function and for the patient in general. These results would be compatible with the hypothesis that activation of the alternative pathway of complement may play an important role in this entity.
Conflict of interestsThe authors declare that they have no potential conflicts of interest in relation with the content of this article.
Please cite this article as: Sánchez-Agesta Martínez M, Rabasco Ruiz C, Sánchez Sánchez R, Ortega Salas R, López Andreu M, Aljama García P. et al. El depósito de C3 en la glomerulonefritis extracapilar de tipo III condiciona un mal pronóstico. Nefrologia. 2018;38:213–219.