The fact that self-locating catheters have a piece of metal at the tip leads to doubt and uncertainty around performing magnetic resonance imaging (MRI) in patients with this type of catheter.
We simulated a peritoneum with a weighted catheter to ascertain how the catheter behaved during MRI scans in 1.5 T and 3 T machines. We also reviewed cases in which MRI had been performed in patients with this type of catheter.
In the simulation, the tip of the self-locating peritoneal catheter caused a magnetic susceptibility artefact that made it difficult to see nearby areas, but it proved to be a safe device for MRI. 14 MRI scans were performed in patients with self-locating catheters, none in the abdominal area. There were no complications in the patients or the technique after performing MRI.
La presencia de una pieza de metal en el extremo de los catéteres autoposicionantes provoca dudas e incertidumbres a la hora de realizar una resonancia magnética (RM) a pacientes que portan este tipo de catéter.
Simulamos un peritoneo con un catéter lastrado para comprobar el comportamiento del catéter durante la realización de una resonancia en equipos 1,5 T y 3 T. Y revisamos los casos en los que se realizaron RM en pacientes con este tipo de catéter.
En la simulación, la punta del catéter peritoneal autoposicionante provoca un artefacto de susceptibilidad magnética que dificulta la visión de zonas cercanas, pero se comporta como dispositivo seguro para la RM. Se realizaron 14 RM en pacientes con catéteres autoposicionantes, ninguna en la zona abdominal. No hubo complicaciones en los pacientes ni en la técnica tras la realización de RM.
Displacement of the catheter tip is a common problem in peritoneal dialysis, and on many occasions a cause of technique failure.
The self-locating catheter, designed to avoid this problem, incorporates a silicone-coated tungsten cylinder at its distal end that helps it to remain in its correct position. The fact that self-locating catheters have a piece of metal at the tip generates doubts and uncertainty about performing magnetic resonance imaging (MRI) in patients with this type of catheter.
According to the American Society for Testing and Materials (ASTM International), the manufacturing company (B. Braun Medical, S.A.) does not specify the safety category of this type of catheter, which is not officially listed as a device that is safe to use during MRI. Nor have we found references in the literature about the compatibility (or lack thereof) of these catheters with MRI.
Given this lack of information, many centres adopt a cautious approach and do not perform this type of diagnostic test on patients with self-locating catheters. However, it is not uncommon that in other centres or situations, due to ignorance of the catheter's characteristics, MRI is performed in these patients.
To clarify these doubts, we decided to simulate a weighted catheter inside a peritoneum to ascertain how the catheter behaves during an MRI. We also reviewed how many of our patients with a self-locating catheter underwent MRI, and if they experienced any problems or complications.
Material and methodsWe initially tested a catheter (Care-Cath SPC. B-Braun) in the MRI equipment. On approaching, the tip exhibited a subtle ferromagnetic effect due to changing magnetic fields at the entrance that exerted a translational force on the catheter tip, drawing it towards the opening of the housing. However, once inside the tunnel, the homogeneous magnetic field did not attract the catheter tip or cause it to move; it remained immobile on the stretcher. This slight effect confirms that the composition of the metal is not 100% homogeneous since tungsten is considered an inert and non-magnetic material.
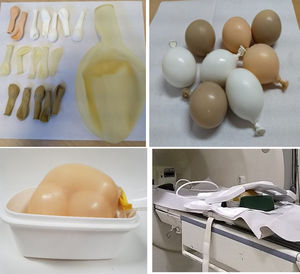
Then, to simulate a peritoneum, we fill several latex balloons of different sizes with water, milk, water with cornflour and milk with cornflour. These balloons were in turn placed inside another large balloon filled with salt water, in which the weighted catheter was also inserted (Fig. 1). We tested our mock peritoneum device in MRI 1.5 T (Horizon GE) and 3 T (Ingenia Philips) equipment, acquiring the locator sequence and T2 single-shot sequences on both pieces of equipment. In the 1.5 T equipment, T2 sequences were also acquired in the three planes, as well as balanced gradient echo (FIESTA). Both the catheter and the balloons were inspected after the tests.
At the same time, we reviewed how many of our patients with self-locating catheters had undergone an MRI study in the last five years, and if they had experienced any type of complication.
ResultsOver the last five years, self-locating catheters have been implanted in 44 patients. Six of them underwent 14 MRIs, most of them craniocerebral (n = 11) for acute pathologies or for monitoring chronic conditions. The remaining MRIs had a trauma indication (n = 2) and a cardiology indication (n = 1). None of the scans concerned the abdominal cavity. Some of the scans were performed in other hospitals or indicated by professionals who were unaware of the nature and composition of the peritoneal catheter. No complications were recorded during or after the procedures.
In our simulation, the position of the catheter remained stable during the scans and its tip did not move during the MRI, nor did it damage or deform the balloons. No heating of the catheter tip was observed after the acquisition of the sequences, which can be explained by the silicone isolation of the metal.
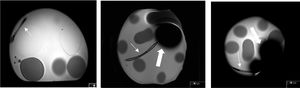
It was observed that the tip of the catheter causes a magnetic susceptibility artifact, with a defect in the signal in the shape of a clover leaf in some sequences. The extension of this artifact, measured in the T2 single-shot sequences, was about 12 cm in 3 T and about 8 cm in 1.5 T. This defect hinders the evaluation of structures that are located in the vicinity of the catheter tip (Fig. 2).
DiscussionDisplacement of the catheter tip is a common problem in peritoneal dialysis, and on many occasions it causes technique failure. The malposition of the tip can be a consequence of its entrapment by the omentum, or be facilitated by intestinal peristalsis and the characteristics/design of the catheter.
The self-locating catheter, designed by di Paolo to avoid this problem, incorporates a silicone-coated tungsten cylinder at its distal end that helps it to remain in its correct position in the pouch of Douglas.1 However, the presence of this piece of metal causes doubts and misgivings when performing an MRI, a diagnostic technique increasingly used in patients who carry this type of catheter, and for an ever-increasing number of indications.
MRI is considered a "safe" technique because it does not involve radiation as it does not use X-rays. However, it is not exempt from other potential risks that may arise as a consequence of the main mechanisms of the functioning of the system2–4:
- -
Displacement force: Due to the strong static magnetic field, any ferromagnetic object can move or accelerate and be attracted to the magnet. This characteristic is responsible for the so-called "projectile effect".
- -
Torsion: The magnetic field acts by aligning the longest axis of the object with the axis of the magnetic field.
- -
Electric currents: To perform the different sequences of an MRI, small magnetic fields, weaker than the main magnetic field, are modified by continuously and rapidly turning them on and off. These changes can induce electric currents in conductive devices and induce neuromuscular stimulation.
- -
Radio-frequency heating: Metal objects can concentrate the energy of radio frequency pulses and thus become hot.
The increasing variety and complexity of medical devices or implants, and the increasingly extensive use and application of MRI, make it necessary to fully understand the behaviour of these objects in the MRI environment. In 1997, ASTM International began to develop methods for evaluating the safety of medical devices and implants in the MRI environment and today, information on the safety of an object or material in MRI is established according to its standards.5–7
This same body has established the definitions by which to classify devices with respect to MRI8:
- -
MR Safe: an item that poses no known hazards in all MR environments.
- -
MR Conditional: an item that poses no known hazards in a specified MR environment with specified conditions for use.
- -
MR Unsafe: an item that is known to pose hazards in all MR environments.
- -
MR not evaluated: this label cannot be used on devices that have any component or proportion of ferromagnetic material in their composition.9
Each definition is accompanied by its corresponding icon, which must appear on the device's packaging, in colour (recommended for greater visibility) or in black and white.
For those devices with the category MR Conditional, the characteristics and conditions in which it is safe to use must also be included (type of generator, power of the magnetic field, types of magnetic field gradients or sequences, etc.).
Most of the tests to determine the safety of a device in MRI have been performed on equipment with 1.5 T power or less. However, the magnetic fields of modern-day equipment are stronger. This can pose a problem for metal objects that are slightly magnetic in 1.5 T equipment but that in more powerful equipment become strongly ferromagnetic.9 Hence the importance of updating compatibility studies for medical devices or implants; the FDA and the ASTM publish standardised guidelines and methods for this verification.5–7,9
The obvious limitation of this study is that we cannot categorically and officially confirm the safety of the self-locating catheter in MRI, given that our simulation was not governed by these standards. Nevertheless, the results of our simulation and the absence of complications in the patients who underwent an MRI scan are reason for optimism in this regard. In our experience, the self-locating catheter is a safe device in MRI and the interference caused by the metal part only affect nearby areas.
However, it is the responsibility of the manufacturing company (which has been informed of our simulation) to demonstrate and clearly indicate the safety of its product, following the established standards.
Conflicts of interestThis study received no specific funding from public, private or non-profit organisations.
Please cite this article as: Moreiras-Plaza M, Nieto-Baltar B, Hernansanz-Pérez M, Carames-Feijoo C, Martínez-Corona E, Lorenzo-García G. Resonancia magnética en pacientes con catéteres peritoneales autoposicionantes: ¿se puede? Nefrologia. 2021;41:41–44.