In December 2019, a coronavirus 2019 (COVID-19) outbreak, caused by SARS-CoV-2, took place in Wuhan and was declared a global pandemic in March 2020 by the World Health Organization (WHO). It is a prominently respiratory infection, with potential cardiological, hematological, gastrointestinal and renal complications. Acute kidney injury (AKI) is found in 0.5%–25% of hospitalized COVID-19 patients and constitutes a negative prognostic factor. Renal damage mechanisms are not completely clear. We report the clinical evolution of hospitalized COVID-19 patients who presented with AKI requiring attention from the Nephrology team in a tertiary hospital in Madrid, Spain.
MethodsThis is an observational prospective study including all COVID-19 cases that required hospitalization and Nephrology management from March 6th to May 12th. We collected clinical and analytical data of baseline characteristics, COVID-19 and AKI evolutions.
ResultsWe analyzed 41 patients with a mean age of 66.8 years (SD 2.1), 90.2% males, and with a history of chronic kidney disease (CKD) in 36.6%. 56.1% of patients presented with sever pneumonia or acute respiratory distress syndrome (ARDS), and 31.7% required intensive care. AKI etiology was prerenal in 61%, acute tubular necrosis in the context of sepsis in 24.4%, glomerular in 7.3% and tubular toxicity in 7.3% of the cases. We reported proteinuria in 88.9% and hematuria in 79.4% of patients. 48.8% of patients required renal replacement therapy (RRT). Median length of stay was 12 days (interquartilic range 9–23) and 22% of the population died. Patients who developed AKI during hospital stay presented with higher C-reactive protein, Lactate dehydrogenase-LDH and d-dimer values, more severe pulmonary damage, more frequent intensive care unit-ICU admission, treatment with lopinavir/ritonavir and biological drugs and RRT requirement.
ConclusionsHypovolemia and dehydration are a frequent cause of AKI among COVID-19 patients. Those who develop AKI during hospitalization display worse prognostic factors in terms of pulmonary damage, renal damage, and analytical findings. We believe that monitorization of renal markers as well as individualized fluid management can play a key role in AKI prevention.
En diciembre de 2019 surgió en Wuhan, China, la COVID-19 causada por SARS-CoV-2, declarada pandemia global por la OMS en marzo de 2020. Es una infección respiratoria con complicaciones a nivel cardiaco, hematológico, digestivo, neurológico y renal. El fracaso renal agudo (FRA) en pacientes hospitalizados por COVID-19 se presenta en el 0,5%–25% y es un factor de mal pronóstico. Los mecanismos de afectación renal no están completamente aclarados. Presentamos la evolución clínica de pacientes ingresados por COVID-19 con FRA que requirieron atención por nefrología en un hospital terciario de la comunidad de Madrid, España.
MétodosÉste es un estudio observacional prospectivo de todos los casos que ingresaron por COVID-19 entre el 6 de marzo y el 12 de mayo de 2020 y requirieron atención por Nefrología. Se recogieron datos clínicos y analíticos de características basales, evolución de la COVID-19 y del FRA.
ResultadosSe analizaron 41 pacientes con edad media de 66,8 años (DE 2,1), el 90,2% varones, y con enfermedad renal crónica previa en el 36,6%. El 56,1% presentaron neumonía grave o síndrome de distrés respiratorio agudo y el 31,7% requirió ingreso en UCI. El FRA fue de etiología prerrenal en el 61%, necrosis tubular aguda en contexto de sepsis en el 24,4%, glomerular en el 7,3% y por toxicidad tubular en el 7,3%. Se registró proteinuria en el 88,9% y hematuria en el 79,4%. El 48,8% de los pacientes requirió terapia de sustitución renal (TSR). La mediana de estancia fue de 12 días (RIC 9–23), y el 22% fallecieron-Los pacientes que desarrollaron FRA durante el ingreso presentaron valores más altos de proteína C-reactiva, LDH o dímero D, una afectación pulmonar más grave, más necesidad de ingreso en UCI, más tratamiento con lopinavir/ritonavir y fármacos biológicos y mayor necesidad de TSR.
ConclusionesLa hipovolemia y deshidratación son una causa frecuente de FRA en pacientes COVID-19. Aquellos que desarrollan FRA intrahospitalario presentan un perfil de peor pronóstico respiratorio, analítico y renal. Creemos que la monitorización de marcadores renales, así como el manejo individualizado de la volemia pueden ser determinantes para prevenir el FRA.
COVID-19, a disease caused by the SARS-CoV-2 coronavirus, emerged in December 2019 in Wuhan, Hubei province, China.1 After a rapid spread, it was declared a global pandemic by the World Health Organization on March 10, 2020. By May 6 there were reported more than 3.5 millions of cases and more than 245,000 deaths worldwide.2 Clinically, COVID-19 affects eminently the respiratory system and triggers an acute respiratory distress syndrome in its most serious forms; involvement at the cardiac, hematological, digestive, neurological and renal levels has also been described.3–5
In the Community of Madrid, between March 9 and May 19, 2020, there were 211,243 suspected cases of COVID-19 registered; 64,410 were confirmed by reverse transcription-polymerase chain reaction (RT-PCR) and there were 34,625 hospitalizations with 2413 admissions to intensive care units (ICU) and 6168 deaths.6
The reported incidence of acute renal failure (ARF) in hospitalized patients ranges between 0.5% and 25%, being even higher in ICUs.3–5,7–9 However, to date we do not have in Spain data on the incidence and characteristics of kidney disease associated with this pandemic. In addition, this impact on COVID-19 patients is not uniform as reported by Chinese hospitals, and may be conditioned by the strategy applied for the case detection of each health system, the policy of patients admissions in each hospital, the definition of kidney injury and even the genetic and environmental factors of the various affected populations.
There is no consensus about the importance of AKI in patients with COVID-19, although it has been associated with higher mortality.9,10 Presently, the etiology of AKI in patients with COVID-19 is being investigated. It has been described a direct involvement of SARS-CoV-2 on renal cells,8,11 and also renal damage secondary to dysfunction of other organs, thrombotic microangiopathy and other more classic systemic causes of renal injury such as hypoperfusion, sepsis or rhabdomyolysis.12,13
In this prospective study, we present the experience of a single nephrology department in a tertiary hospital in Madrid (Spain), with a complete portfolio of services and hematopoietic and solid organ transplantation programs, which serves a population of half a million of people. From February 26 to April 26, there were 59,129 cases of COVID-19 diagnosed in the Community of Madrid and 7922 of these affected patients died. During the same period, 1603 admissions for COVID-19 were registered in our hospital, and the mortality in the hospital was 12.3%.14 Our objective is to describe the different presentations of AKI that required medical attention by nephrologists, their clinical evolution and possible strategies for early detection and kidney protection.
MethodsStudy design and participantsWe carried out a consecutive systematic sampling of all the cases of COVID-19 in adults admitted to the Puerta de Hierro Majadahonda University Hospital who required attention from the Nephrology department due to deterioration of kidney function between March 6 and May 12, 2020. The diagnosis of COVID-19 was based on the clinical and radiological criteria established by the World Health Organization and was confirmed by the detection of SARS-CoV-2 in nasopharyngeal exudate by means of RT-PCR.15 Oral consent was obtained from the patients for the use of treatments outside the indication in the data sheet and for the analysis of their clinical data, being reflected in the electronic clinical history. This study was approved by the Research Ethics Committee of the Puerta de Hierro Majadahonda University Hospital (IRB Number 88/20).
Data collection and definitionsThe analysis included all cases with outcome (exitus or hospital discharge ) and those hospitalized patients that had been monitored at the least 4 weeks. The clinical and analytical data were collected prospectively from the hospital's electronic medical record into a specially designed database. It includes demographic data, comorbidity, aspects of COVID-19 and its specific treatment (lopinavir/ritonavir, hydroxychloroquine, azithromycin, corticosteroids, biological agents such as interferon, tocilizumab or anakinra), analytical data of potential prognostic value,16 etiology and evolution of kidney damage, days of hospitalization and clinical outcomes.
The severity of the SARS-CoV-2 infection was determined according to the World Health Organization classification for COVID-19 and the CURB-65 scale,15,17 severe lung damage was considered if the patient had severe pneumonia and acute respiratory distress. Mechanical ventilation includes invasive and non-invasive ventilation. The normal laboratory values were the reference values indicated by hospital laboratory. AKI was classified into 3 stages according to the KDIGOguidelines,18 considering the highest value of serum creatinine recorded during admission. Proteinuria was considered if greater 0,2 g/L in the urine strip and hematuria was the presence of at least 2–5 red cells/field in the urine sediment. The etiology of the AKI was determined using standard clinical diagnostic algorithms by nephrology specialists. Renal replacement therapy (RRT) techniques were categorized into conventional hemodialysis and continuous veno-venous hemodiafiltration. Patients diagnosed of AKI in the emergency room were classified as “AKI on admission”, and “in-hospital AKI” those developed AKI during the hospital stay.
StatisticsContinuous variables were expressed as means (standard deviation) or medians (interquartile range) and were compared with the Student's t-test or the Mann-Whitney U test. The categorical variables were expressed in percentages and were compared using the Chi-square test. Statistical analysis was performed with Stata (version 14.1), accepting P < .05 as statistical significance.
ResultsBaseline characteristicsThere were 41 patients included. The baseline characteristics are shown in Table 1. A 90.2% were male and the mean age was 66.8 years. Some degree of previous chronic kidney disease or kidney transplants was present 36.6% of patients. Diabetes mellitus, obesity, arterial hypertension and COPD were observed in 39,0, 26.8, 73.2 and 14.6% of the cases, respectively. The median period of time elapsed since the onset of symptoms to the arrival at the emergency room was 7 days (interquartile range 2–8).
Baseline characteristics of COVID-19 patients admitted with or AKF.
| Total | AKF at admission | AKF during Hospital admission | P | |
|---|---|---|---|---|
| n | 41 | 23 | 18 | |
| Age (years), mean ± SD | 66,8 ± 2,1 | 67,4 ± 15,6 | 66,0 ± 10,4 | ,7 |
| Male n (%) | 37 (90.2) | 21 (91.3) | 16 (88.9) | ,8 |
| HTN, n (%) | 30 (73.2) | 17 (73.9) | 13 (72.2) | ,9 |
| DM, n (%) | 16 (39) | 8 (34,8) | 8 (44,4) | ,5 |
| Obesity, n (%) | 11 (26,8) | 6 (26,1) | 5 (27,8) | ,9 |
| COPD, n (%) | 6 (14,6) | 3 (13,0 ) | 3 (16,7) | ,7 |
| Previous CKD, n (%) | 15 (36,6) | 9 (39,1) | 6 (33,3) | ,9 |
| Delay a (days), median [IQR] | 7 [2–8] | 5 [2–7] | 7 [3–12] | ,5 |
SD: standard deviation; DM: diabetes mellitus; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; ARF : acute renal failure; HTN: arterial hypertension; IQR: interquartile range.
The data on the SARS-CoV-2 infection are shown in Table 2. A 56.1% had severe pneumonia or acute respiratory distress syndrome, with a median CURB-65 of 2 (interquartile range 2–5). A 70.7% required mechanical ventilation and 31.7% had to be admitted in ICU. Diarrhea was reported in 48.8% of the cases. Patients that developed AKI after hospital admission presented greater clinical severity and showed higher ferritin, C-reactive protein, IL-6, LDH and d-dimer values than patients who were admitted with evidence of AKI (Table 2). Regarding treatment, Lopinavir/ritonavir was used in 51.2% of the patients, hydroxychloroquine in 95.1%, azithromycin in 65.9%, corticosteroids in 80.5%, and biological agents in 51.2%.
Clinical evolution of patients admitted with COVID-19 infection.
| Total | AKF at admission | AKF during Hospital admission | P | |
|---|---|---|---|---|
| n | 41 | 23 | 18 | |
| Respiratory | ||||
| Scale CURB-65 medium [RIC] | 2 [2–5] | 2 [2–3] | 3.5 [1–5] | ,8 |
| ARDS/severe pneumonia, n (%) | 23 (56,1) | 9 (39,1) | 14 (77,8) | ,01 |
| Ventilation MECAN ica, n (%) | 29 (70,7) | 15 (6,.2) | 14 (77,8) | ,01 |
| ICU admission, n (%) | 13 (31,7) | 3 (13) | 10 (55,6) | ,004 |
| Diarrhea, n (%) | 20 (48,8) | 13 (5,5) | 7 (38,9) | ,3 |
| Analytical data, median [IQR] | ||||
| Minimum lymphopenia (×103/μl) | 310 [200−460] | 330 [226−650] | 265 [170−370 ] | ,1 |
| Maximum CRP (mg/l) | 183 [95−250] | 124 [49,8−237] | 250 [124−250] | ,01 |
| Dimer or d-max (μg/mL) | 3,6 [1.8−10] | 2,53 [0.9−7,7] | 7.2 [3.3−11.7] | ,02 |
| Maximum ferritin (ng / ml) | 1204 [473−2326] | 1192 [473−2108] | 1586 [504−3169] | ,6 |
| Maximum LDH (U / l) | 503 [ 372−617] | 399 [296−545] | 573 [514−640] | ,03 |
| Maximum IL-6 (pg / ml) | 57,3 [19,5−467] | 48,3 [15,9−109] | 2749 [44−650] | ,08 |
| Drugs, n (%) | ||||
| Lopinavir / ritonavir | 21 (51,2) | 4 (17,4) | 17 (94,4) | <,001 |
| Hydroxychloroquine | 39 (95,1) | 21 (91,3) | 18 (100) | ,2 |
| Azitr omicina | 27 (65,9) | 15 (65,2) | 12 (66,7) | ,9 |
| Corticosteroids | 33 (80,5) | 17 (73,9) | 16 (88,9) | ,2 |
| Biological | 21 (51,2) | 7 (30,3) | 14 (77,8) | ,003 |
| Hospital stay (days), median [IQR] | 12 [9–23] | 11 [9–22] | 22 [9–24] | ,3 |
| Outcome, n (%) | ,6 | |||
| Exitus | 9 (22) | 5 (21,7) | 4 (22,2) | |
| High without RRT | 23 (56,1) | 17 (73,9) | 7 (38,9) | |
| High with RRT | 1 (2,4) | 0 | 1 (5,5) | |
| Hospitalized | 8 (19,5) | 2 (8,7) | 6 (33,3) |
SD: standard deviation; AKF: acute renal failure; IL-6: interleukin 6; LDH: lactate dehydrogenase; CRP: C-reactive protein; IQR: interquartile range; ARDS: acute respiratory distress syndrome; RRT: renal replacement therapy; ICU: Intensive Care Unit.
In bold, statistically significant differences.
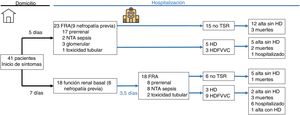
The presentation and evolution of AKI is shown in Table 3. A 56.1% of the cases had acute deterioration of renal function upon arrival at the Emergency Department, while the rest developed AKF during admission. The causes of AKF were prerenal in 61% of cases, secondary to acute tubular necrosis (ATN) in the context of sepsis in 24.4%, tubular toxicity in 7.3%, and glomerular origin in 7.3%. Proteinuria > 0.2 g/L was present in 88.9% of cases and hematuria by dipstick was observed in 79.4% of patients. In 39% of cases, urine was collected after bladder catheterization. The mean maximum creatinine and urea were 4.0 and 205 mg/dl, respectively. A 12.2% were AKF grade 1, 7.3% grade 2, and 80.5% grade 3. Some type of RRT had to be used in 48.8% of the cases, with a mean of 5 treatment sessions. A 56.1% of the cases were registered during the first 20 day period since the initiation of the study. In this first period, 83.3%15 were ARFs developed after hospital admission and 34.8%8 of ARFs were present at the time of admission.
Characteristics of acute renal failure in patients admitted for COVID-19.
| Total | AKF at admission | AKF during Hospital admission | P | |
|---|---|---|---|---|
| n | 41 | 2,3 | 18 | |
| Etiology,% | ,02 | |||
| Prerenal | 61,0 | 73,9 | 44,4 | |
| ATN sepsis | 24,4 | 8,8 | 44,4 | |
| Glomerular | 7,3 | 13 | 0 | |
| Tubular toxicity | 7,3 | 4,3 | 11,2 | |
| Urine strip,% | ||||
| Proteinuria | 88,9 | 94,7 | 82,4 | ,5 |
| Hematuria | 79,4 | 70 | 92,9 | ,1 |
| AKIN,% | ,6 | |||
| Grade 1 | 12,2 | 13 | 11,1 | |
| Grade 2 | 7,3 | 13 | 0 | |
| Grade 3 | 80,5 | 74 | 88,9 | |
| Biochemical data, median [IQR] | ||||
| Initial Cr (mg/dl) | 1,73 [1–3,1] | 2,34 [1,4–3,7] | 1,11 [0.8–1.36] | ,00 |
| Maximum Cr (mg/dl) | 3,62 [2,33–5,5] | 3,27 [2,33–5,21] | 3,79 [2.24–5.55] | ,9 |
| Initial urea (mg/dl) | 77 [45–153] | 124 [72–188] | 60 [40–72] | ,00 |
| Maximum urea (mg/dl) | 199 [122–279] | 161 [111–264] | 203 [122–289] | ,5 |
| RRT,% | 48,8 | 34,8 | 66,7 | ,04 |
| Conventional HD | 19,5 | 21,7 | 16,7 | |
| CVVHDF | 29,3 | 13 | 50 |
AKIN: Acute Kidney Injury Network; Cr: creatinine; SD: standard deviation; AKF: acute kidney failure; HD: hemodialysis; HDFVVC: continuous veno-venous hemodiafiltration; ATN: acute tubular necrosis; IQR: interquartile range; RRT: renal replacement therapy.
In bold, statistically significant differences.
Comparison of data from patients with “ARF on admission” and “in-hospital ARF” is summarized in Tables 1–3 and in Fig. 1. The in-hospital ARF group had a higher incidence of ATN in the context of sepsis (8,8 vs. 44,4; P = ,008), higher maximum C-reactive protein (124 vs. 250; P = ,01), higher maximum LDH (399 vs. 573; P = ,027), greater d-dimer (2.53 vs. 7.20; P = ,015), more severe lung involvement (39,1 vs. 77,8; P = ,013), more need for mechanical ventilation (65,2 vs. 77,8; P = ,009), more need for ICU admission (13,0 vs. 55,6; P = ,004), more use of lopinavir/ritonavir (17,4 vs. 94, 4; P = ,0001) and biological drugs (30,3 vs. 77,8; P = ,003), and greater need for RRT (34,8 vs. 66,7; P = ,043).
Clinical outcomeThe mean period of hospitalization was 15.8 days, with a minimum follow-up time for those patients who were still hospitalized for 4 weeks. At the end of the study, 22% of the patients had died, 56% had been discharged without the need for RRT, 2.4% required RRT after discharge and 19.5% remained hospitalized (Fig. 1).
DiscussionThis study is the first to analyze a Spanish cohort of AKF associated with COVID-19 followed by nephrologists and describes a wide variety of situations of kidney damage that go beyond a plain direct parenchymal damaged induced by the SARS-CoV-2 virus. Most available studies come from hospitals in the Wuhan area, where the pandemic8–10 was originated, and the references focus on the management of patients undergoing chronic RRT.19
The main publications of AKF in patients with SARS-CoV-2 focus on renal histopathology, describing the damage as a result of the direct cellular damage caused by the virus or the secondary “cytokines storm”.13 However, other classical pathophysiological factors of conventional AKF appear to be ignored. Our study found a high incidence of severe prerenal AKF, which is present in almost 50% of the cases and is two fold of the values previously reported by the Madrid studio GEFRAM in a situation free of pandemic.20 Diarrhea is a relevant factor for dehydration in these patients, although until now it had been considered a symptom present in only in 3%–10% of the SARS-CoV-2 infection3,4,21; diarrhea is 10 times higher in our series, although it should not be forgotten that it is a selection of patients with renal involvement. It is also striking to observe that patients waited one week to came to the emergency room after first symptoms appeared, similar to what it was reported by Cheng et al.9 This situation could be due to a combination of factors such as a saturated healthcare system, fear to attend to a hospital, and the initial recommendations to stay at home issued by the health authorities.
In the early stages of the pandemic there were published documents clinical that recommended a conservative use of fluid therapy to prevent pulmonary edema.22,23 Although it is true that COVID-19 infection does not usually present in a state of shock that requires resuscitation with volume,24 hypovolemia may develop with factors that favor insensitive losses such as fever, gastrointestinal losses and even limited access to water due to extreme isolation and lack of companions in the room. It is striking that the incidence of prerenal AKF is not evenly distributed throughout the study period, since most of our cases were registered during the first 20 days of the study. This coincides with the modification of the hospital protocol that initially was more conservative with the use of fluid therapy and promoted the generalized use of lopinavir / ritonavir with its consequent predisposition to diarrhea; in fact, 83.3% of hospital ARFs were in this first period.
The clinical presentation of AKF varied, and only 3 cases (7.3%) were associated with glomerular diseases. A case of Lupus nephropathy was detected that was previously unknown and there were 2 cases of thrombotic microangiopathy, the latter being inherent to renal pathophysiology described in SARS-CoV- 2 infection.13 Also, both proteinuria and hematuria have been described as factors independent associated with higher hospital mortality in these patients.9 In our study, we found that the frequency of proteinuria and hematuria was twice as much as that reported by Li et al. in 193 cases,10 and up to three times the values described by Cheng et al. in 701 cases,9 although part of the samples were taken after bladder catheterization.
We have observed 2 types of patients with AKF clearly differentiated: the group with AKF on admission and the in-hospital AKF group. Both groups had a similar demographic and comorbidity profile (Table 1); However, the in-hospital AKF group presents a significantly higher proportion of ATN associated with sepsis, while the AKF group at admission is mainly associated with pre-renal factors (Table 3). In general, patients that developed AKF in the hospital had a more severe disease with intense pulmonary involvement, need for mechanical ventilation, more admissions to the ICU and analytical parameters that indicate a worse prognosis (Table 2).21,25 The AKF was more severe in this group and required more time of RRT.
The presence of AKF is a factor of a worse prognosis and higher mortality in patients admitted with SARS-CoV-2 infection.9,10 Mortality in this study was 22%, similar to or estimated in the population with kidney disease by Cheng et al.9
This work is, in our opinion, the first one specifically designed to evaluate the development of ARF in patients with SARS-CoV-2 infection in Spain and the first that analyzes the different clinical patterns, highlighting the importance of prerenal ARF in this pandemic. However, it has limitations, such as the sample size, a negative selection of the most severe cases, and the lack of renal biopsy.
The scenario of the COVID pandemic is changing rapidly. When this study was performed, the incidence was decreasing which serve to alleviate the care burden, there is more availability of serological tests and RT-PCR, and we have more evidence to adjust the treatments. This means that patients go to the emergency room earlier and are treated since the early stages of the disease. The protocols have evolved and aiming a global approach of the patient, which includes complications in its evolution, such as ARF. The national epidemiological study has only identified 5% exposure to the virus in the general population (11% in Madrid).26 Finally, we are faced with a new profile of patients who are admitted for any reason and associate a SARS-CoV-2 infection detected by an incidental RT-PCR. The experts' impression is that COVID-19 pandemic will last thus COVID-19 patients will be with us for months and all specialists should monitor kidney involvement and protect the patient from the risk of ARF if the long- term consequences of this pandemic are to be minimized.
In conclusion, ARF occurs in COVID-19 with varied clinical expression; it stand out the prerenal cause of kidney failure and ATN associated with sepsis. Frequent monitoring of kidney damage markers, as well as individualized management of blood volume, may be decisive in preventing AKF, especially in patients with more serious infections.
FinancingStudy co-financed by an “Unrestricted Grant” from FRIAT through the Madrid Nephrology Foundation and the Segovia de Arana-Puerta de Hierro-Majadahonda Research Institute, Madrid (018/02FRA).
Conflict of interestsThe authors declare that they have no conflict of interest.
ThanksWe are grateful for the technical assistance of Dr. Paula López Sánchez, BSc, PhD, in the methodological aspects and statistical analysis.
Please cite this article as: Tarragón B, Valdenebro M, Serrano ML, Maroto A, Llópez-Carratalá MR, Ramos A, et al. Fracaso renal agudo en pacientes hospitalizados por COVID-19. Nefrologia. 2021;41:34–40.