Alterations in bone and mineral metabolism are very common in chronic kidney disease (CKD). The increase in phosphate levels leads to bone disease, risk of calcification and greater mortality, so any strategy aimed at reducing them should be welcomed. The latest drug incorporated into the therapeutic arsenal to treat hyperphosphataemia in CKD is Sucroferric Oxyhydroxide (SFO).
ObjectiveTo analyse the efficacy and safety of OSF in three cohorts of patients, one with advanced chronic kidney disease not on dialysis (CKD-NoD), another on peritoneal dialysis (PD) and the last on haemodialysis (HD), followed for six months.
MethodsA prospective, observational, multicentre study in clinical practice. Clinical and epidemiological variables were analysed. The evolution of parameters relating to alterations in bone and mineral metabolism and anaemia was analysed.
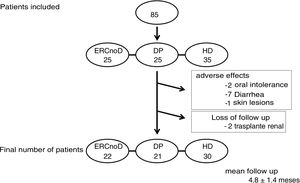
ResultsEighty-five patients were included in the study (62 ± 12 years, 64% male, 34% diabetic), 25 with CKD-NoD, 25 on PD and lastly, 35 on HD. In 66 patients (78%), SFO was the first phosphate binder; in the other 19, SFO replaced a previous phosphate binder due to poor tolerance or efficacy. The initial dose of SFO was 964 ± 323 mg/day. Overall, serum phosphate levels saw a significant reduction at three months of treatment (19.6%, P < 0.001). There were no differences in the efficacy of the drug when the different populations analysed were compared. Over the course of the study, there were no changes to levels of calcium, PTHi, ferritin, or the transferrin and haemoglobin saturation indices, although there was a tendency for the last two to increase. Twelve patients (14%) withdrew from follow-up, ten due to gastrointestinal adverse effects (primarily diarrhoea) and two were lost to follow-up (kidney transplant). The mean dose of the drug that the patients received increased over time, up to 1147 ± 371 mg/day.
ConclusionsSFO is an effective option for the treatment of hyperphosphataemia in patients with CKD both in the advanced phases of the disease and on dialysis. We found similar efficacy across the three groups analysed. The higher their baseline phosphate level, the greater the reduction in the serum levels. A notable reduction in phosphate levels can be achieved with doses of around 1000 mg/day. Diarrhoea was the most common side effect, although it generally was not significant.
Las alteraciones del metabolismo óseo y mineral son muy frecuentes en la enfermedad renal crónica (ERC). El aumento en los niveles de fósforo condiciona enfermedad ósea, riego de calcificación y mayor mortalidad por lo que cualquier estrategia encaminada a su reducción debe ser bienvenida. El último fármaco incorporado al arsenal terapéutico para tratar la hiperfosforemia en la ERC es el Oxihidróxido sucroférrico (OSF).
ObjetivoAnalizar la eficacia y seguridad de OSF en 3 cohortes de pacientes, una con enfermedad renal crónica avanzada no en diálisis (ERCnoD) otra en diálisis peritoneal (DP) y finalmente otra en hemodiálisis (HD), seguidas durante 6 meses.
MétodosEstudio observacional multicéntrico, prospectivo, de práctica clínica. Se analizaron variables clínicas y epidemiológicas. Se valoró la evolución de parámetros relacionados con las alteraciones del metabolismo óseo y mineral y la anemia.
ResultadosSe incluyeron en el estudio 85 pacientes (62 ± 12 años, 64% varones, 34% diabéticos), 25 con ERCnoD, 25 en DP y finalmente 35 en HD. En 66 pacientes (78%) OSF fue el primer captor del fósforo; en los otros 19 se sustituyó un captor previo por OSF, por falta de tolerancia o eficacia. La dosis inicial de OSF fue 964 ± 323 mg/día. Globalmente los niveles séricos de fósforo experimentaron un descenso significativo a los 3 meses de tratamiento (19,6%, P < 0,001). No hubo diferencias en la eficacia del fármaco al comparar las distintas poblaciones analizadas. A lo largo del estudio no se modificaron los niveles de calcio, PTHi, ferritina, índice de saturación de la transferrina ni hemoglobina, aunque se manifestó una tendencia al aumento de los dos últimos. Doce pacientes (14%), abandonaron el seguimiento, 10 por efectos adversos gastrointestinales (diarrea fundamentalmente) y 2 por pérdida de seguimiento (trasplante renal). La dosis media del fármaco que recibieron los pacientes se incrementó a lo largo del tiempo hasta alcanzar los 1147 ± 371 mg/día.
ConclusionesOSF es una opción eficaz para el tratamiento de la hiperfosforemia en pacientes con ERC tanto en fases avanzadas de la enfermedad como en diálisis. Encontramos una eficacia similar en los 3 grupos analizados. A mayor nivel basal de fósforo, mayor descenso de sus niveles séricos. Con dosis alrededor de 1000 gr/día se puede conseguir un notable descenso de los niveles de fósforo. La diarrea fue el efecto secundario más frecuente, aunque generalmente fue poco importante.
In chronic kidney disease (CKD), patients the abnormalities in bone and mineral metabolism are very common and become apparent even in its early stages of the disease. An increase in the concentration of fibroblast growth factor 23 and a decrease in Klotho1 have been described in patients with moderate decreases in glomerular filtration. With the phosphaturic effect of fibroblast growth factor 23 the serum phosphorus levels remain in the normal range in those first stages of kidney disease. However, as kidney disease progresses, the ability to eliminate the load of phosphorus, which mainly comes from the diet, is reduced. Hyperphosphataemia usually develops when the glomerular filtration rate falls below 30 ml/min2,2 and appears to be involved in the progression of renal disease itself3 and the development of vascular calcification in patients without kidney disease.4 The control of serum phosphorus levels has always been the object of great concern for nephrologists who care for patients with CKD. A direct association between elevated phosphorus levels and mortality has been demonstrated in numerous observational studies in dialysis and predialysis patients.5–10 Given this situation, any strategy aiming to reduce serum phosphorus levels in uremic patients should be welcomed. Thus, it has been reported the beneficial effects of low phosphorus diets,11 modifications in dialysis regimens to achieve greater extrarenal clearance of phosphorus,12 or the use of intestinal phosphate binders13 to reduce its absorption. It has also been seen that the use of drugs that lower parathyroid hormone (PTH), as cinacalcet, are able to reduce serum phosphate levels, probably due to an improvement in bone remodeling.14 All of these therapies have been associated with a reduction in the risk of mortality.
Currently marketed phosphorus binders are divided into those that contain calcium and those without calcium in their composition. The latest guidelines from Kidney Disease: Improving Global Outcomes, from 2017, recommend restricting the use of calcium-based phosphorus binders because they contribute to the positive calcium balance in patients with CKD and increase the risk of soft tissue calcification.15 Sucroferric oxyhydroxide (OSF) is a new phosphorus binder with iron that is indicated in adult patients with CKD. In addition to pre-marketing studies,16 some clinical studies are a being published in both hemodialysis (HD) and peritoneal dialysis (PD) patients. In all cases, a clear reduction in serum phosphorus levels is observed. This effect is observed both in patients in whom OSF is the first used captor and in those in which ODF is the substitution of another.17–19 There are no published data on the effect of this drug in patients in advanced stages of CKD who have not yet required dialysis.
The main objective of the present study was to analyze the efficacy and safety of OSF in 3 cohorts of patients: one with advanced CKD not on dialysis, another on PD and finally another on HD, followed up for 6 months.
MethodsThis is a multicenter, prospective, observational study of clinical practice carried out in the nephrology services of 4 public hospitals in our region. All patients who receiving OSF for hyperphosphataemia were evaluated. The patients came from the PD, HD programs and advanced CKD outpatient clinics (all of them with glomerular filtrations less than 30 ml/min).
Inclusion criteria were: 1) patients of both sexes, older than 18 years; 2) diagnosed with CKD and hyperphosphataemia (defined as a serum phosphorus concentration above 4.5 mg/dl), and 3) with no known reaction or possible intolerance to the study drug. Patients with known poor adherence to treatment were excluded.
The study was approved by the Ethics Committee and the participating patients signed an informed consent to join the study.
ProceduresThe intervention consisted in oral administration of OSF for 6 months. As it was a clinical practice study, the doctor responsible for the patient decided the initial dose of the drug based mainly on the phosphorus concentration at that time. Subsequent variations in dosage were based on the evolution of serum phosphorus values. If the desired decrease was not achieved, the OSF dose was increased. The patients were instructed to take the drug during meals.
Patients who were previously receiving another phosphorus binder were subjected to a 2-week washout period to avoid interferences that could alter the results. As an additional measure and before starting the study, all patients were informed that they suffered from hyperphosphataemia and the risks that this entails. The possible adverse effects that the drug could have were also explained to the patients, such as the blackish coloration of the stool or the increase of the intestinal rhythm and the lower consistency of the stool. Those patients who did not complete the study period for any reason were analyzed by intention to treat.
Biochemical determinationsThe general biochemistry, including serum calcium and phosphorus levels, were determined with an autoanalyzer, following the procedures of the hospital laboratory. The calcium values reported in the present study are expressed as total calcium uncorrected by serum protein or albumin levels. The determination of PTH was performed by electrochemiluminescence (EQL Elecsys ® PTH, by Ro che) and the results were corrected with the coefficient 0.97 to express them as IRMA, by Nichols, according to the K/DOQI20 guidelines. Renal function was determined by creatinine clearance using 24 h urine collection.
Statistical analysisContinuous variables were expressed as mean and standard deviation and categorical variables as percentage. The KoImogorov-Smirnov test had previously been used to determine if the data followed a normal distribution. Those variables that did not show a normal distribution were expressed as median and interquartile range. To analyze the evolution of the serum levels of the different variables during the study period, the general linear model with repeated measures was used. To know the best predictors of response to treatment, a linear regression model was applied. The SPSS ® 20 statistical package for Windows (SPSS Inc., Chicago, IL) was used to analyze of the results.
ResultsFinally, 85 patients were included in the study, of which 35 were on HD, 25 on PD and the rest were followed in the advanced CKD outpatient clinic (not on dialysis). The characteristics of the patients are shown in Table 1. The 3 groups of patients had similar characteristics, except for a slightly older age in those who were on HD. In 66 patients (78%) OSF was the first phosphorus binder prescribed to treat recent diagnosis of hyperphosphataemia; in the other 19 patients, another previous captor was replaced by OSF, due to lack of tolerance or efficacy. The initial dose of OSF was 964 ± 323 mg/day, which is below the recommended doses indicated in the data sheet of the product.
Baseline characteristics of the patients. Initial and final dose of sucroferric oxyhydroxide.
| All | CKD-not on D | PD | HD | |
|---|---|---|---|---|
| N | 85 | 25 | 25 | 35 |
| Age (years), mean ± SD | 62 ± 12 | 60 ± 15 | 61 ± 14 | 64 ± 16 |
| Sex (% males) | 64 | 61 | 64 | 63 |
| Diabetics (%) | 34 | 32 | 35 | 34 |
| New treatment (%) | 78 | 94 | 74 | 71 |
| Initial dose OSF (mg/dl), mean ± SD | 964 ± 323 | 750 ± 128 | 955 ± 206 | 1.125 ± 358 |
| Final OSF dose (mg/dl), mean ± SD | 1,147 ± 371 | 876 ± 185 | 1,093 ± 222 | 1,384 ± 411 |
SD: Standard Deviation; PD: Peritoneal Dialysis; CKD not on D: Chronic Kidney Disease not on dialysis; HD: Hemodialysis; OSF: Sucroferric Oxyhydroxide.
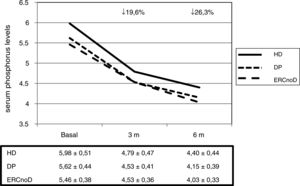
Overall, serum phosphorus levels experienced a significant decrease after 3 and 6 months of treatment; from 5,7 ± 0,5 to 4,6 ± 0,4 and 4,3 ± 0,4 mg/dl, respectively (P < 0,001). The mean decrease was 19,6% at 3 months and reached 26,3% at month 6. With this reduction in serum phosphorus concentration, 68% of patients were in the normal range at 3 months and 83% at 6 months, according to the reference values published by the latest guidelines of the Spanish Society of Nephrology.20
There were no differences in the efficacy of the drug in the different populations analyzed (Fig. 1). There were also no differences in relation to those patients in whom OSF was the first captor or those in whom it was a change from a previous one.
In the univariate analysis, we observed that the percentage of decrease in serum phosphorus with respect to baseline after 6 months of treatment was directly associated with basal phosphorus levels (r = 0,473; P < 0,001). Other demographic and biochemical parameters had no effect. In the multivariate linear regression model, only the baseline phosphorus level (Exp [B] = 0,87 [95% CI 0,74-0,94]; P < 0,001) was an independent predictors of the response to treatment.
Throughout the study, the serum levels of calcium, iPTH, ferritin, transferrin saturation index, or hemoglobin did not change significantly, although there was an increasing trend in the last 2 parameters (Table 2).
Evolution of analytical parameters.
| Basal | 3 months | 6 months | p | |
|---|---|---|---|---|
| Ca (mg/dl), mean ± SD | 9.2 ± 0.6 | 9,1 ± 0,5 | 9.1 ± 0.6 | 0.150 |
| P (mg/dl), mean ± SD | 5,7 ± 0.5 | 4,6 ± 0,4 | 4,3 ± 0,4 | <0,001 |
| PTH (pg/ml), median (IQR) | 305 (356) | 292 (331) | 318 (345) | 0.384 |
| Hemoglobin (g/dl), mean ± S D | 11,1 ± 1,2 | 11,3 ± 1,2 | 11,4 ± 1, 4 | 0,089 |
| Ferritin (ng/ml), mean ± SD | 177 ± 56 | 186 ± 71 | 194 ± 77 | 0,101 |
| TSI (%), mean ± SD | 18,1 ± 2.3 | 19.3 ± 2.8 | 19.8 ± 3.1 | 0,092 |
Ca: Calcium; SD: Standard Deviation; IST: Transferrin Saturation Index; P: Phosphorus; PTH: Parathyroid hormone; IQR: Interquartile range.
The average dose of the drug received increased throughout the followup until 1.147 ± 371 mg/day aiming to achieve the control of serum phosphorus levels.
Twelve patients (14%) abandoned the follow-up, 10 due to gastrointestinal adverse effects (mainly diarrhea) and 2 due to loss of follow-up (kidney transplantation) (Fig. 2). The mean follow-up of the total sample of patients was 4,8 ± 1,1 months.
DiscussionThe present study confirms the efficacy of OSF in controlling phosphorus levels in patients with CKD both on dialysis and in those with CKD in the predialysis stage.
Under normal conditions, 90% of phosphorus ingested is excreted in the urine with the remaining 10% is eliminated in the faeces.21 When kidney disease progresses, the reduced number functioning nephrons will not be able to filtrate enough phosphorus and plasma levels will increase progressively.22 Conventional dialysis techniques are unable to eliminate this excess phosphorus, although it has been described that the use of convective techniques23 or frequent HD24 can help normalize it without requiring the use of drugs. For all this, in most cases it will be necessary to prescribe intestinal binders to try to normalize phosphataemia.
Calcium-based phosphorous binders are generally well tolerated, effective, and inexpensive; however, clinical practice guidelines15 recommend not using them in the case of previously diagnosed hypercalcemia or vascular calcification. And we must admit that many patients with advanced CKD are calcified and the percent of patients with calcifications in HD and PD is 90% and 80% respectively.25,26 Therefore the need to use non-calcium-based captors arose years ago. Sevelamer and lanthanum are 2 commonly used non-calcium binders.
The efficacy of non-calcium phosphorus binders has been widely analyzed. It is estimated that with these binders the reduction of phosphataemia in the different treatment groups is around 25%. Sevelamer has been shown to reduce serum phosphorus levels by around 20% after 8 weeks of treatment in CKD patients not on dialysis27; in close to 200 HD patients after 46 weeks the decrease in serum P was 25%28; finally, in PD patients after 12 weeks the decline in phosphorus was about 22%.29 Similar results are observed with lanthanum the; serum phosphorus levels decreased around 25% in just 4 weeks of treatment in CKD patients not on dialysis,30 in HD patients after 36 months of treatment the serum phosphorus decreased a 21%31; and in PD patients the serum phosphorus decreased a 24 after 48 weeks of treatment.32 The efficacy of sevelamer and lanthanum was similar33 although an additional advantage of sevelamer is the pleiotropic effects (improvement in lipid profile and uric acid levels, increased alkaline phosphatase, reduction in C - reactive protein).34 Despite these therapeutic options, half of the patients on HD and PD do not reach the desired goals recommended by the guidelines.35 Failure to achieve the objectives may be explained by different factors including polypharmacy (a patient on HD can take 20 tablets a day, half of them being phosphorus binders), and the adverse effects, most of them gastrointestinal, which lead to patients to reduce doses or abandon treatments.36 Also the eating habits of frequent consumption of precooked or preserved products rich in inorganic phosphorus worsens the situation.37
With the avenue of OSF it was shown that among the advantages of this drug are its efficacy, the few number of tablets required, and the adequate gastrointestinal tolerance. These effects have been confirmed in the different studies published so far. Before commercialization, clinical trials were performed to assess the efficacy and safety of this drug in dialysis patients. Floege et al.16 published the results of 644 dialysis patients (91% on HD, 9% on PD), with hyperphosphataemia (defined as serum phosphorus greater than 6 mg/dl), randomized to receive sevelamer carbohydrate or OSF with the objective to reach phosphorus levels between 2.5 and 5.5 mg/dl. In this study, it was found that OSF is as effective as sevelamer in reducing phosphataemia and the same number of patients achieved the objectives recommended by the guidelines. The mean decrease in phosphataemia achieved was 25% at 3 months, and the values remaining stable until 52 weeks of follow-up. A highlight of this study is the reduced the number OSF tablets required to achieve the phosphorus target.
Subsequently, different real-life studies have been published that analyzed the efficacy of OSF in dialysis patients. Kendrick et al.18 analyzed the achievement of serum phosphorus targets after substitution of different binders (calcium acetate, sevelamer or lanthanum). The study concluded that with OSF the possibility of having serum phosphorus levels within the range recommended by the guidelines is twice that of other binders. Coyne et al.17 followed the evolution of more than 1000 prevalent HD patients treated with OSF. This drug achieved a 12% increase in the number of patients reaching the recommended serum phosphorus targets. Finally, Kalantar-Zadeh et al. analyzed the evolution of serum phosphorus in a cohort of 258 PD patients that were prescribed OSF, achieving an overall decrease in phosphatemia and almost 3 out of 4 patients achieved target phosphorus indicated in the guides.19 To date, there are no published data on the effect of OSF on serum phosphorus in patients with CKD who are not on dialysis, so our work will be the first to provide information in this regard.
The mean decrease in serum phosphorus levels in our study was 20% at 3 months and 26% at 6 months. A differential aspect with respect to the clinical trial published by Floege et al.16 is that in our study the serum phosphorus continued to fall until 26 weeks whereas in the Floge study the phosphatemia continue to decrease 12 weeks.
Usually no many PD patients are included in drug efficacy studies. In our case, we wanted to take this into consideration. We did not find statistically significant differences in the baseline phosphorus levels of the PD vs HD patients, and the percent decrease in phosphataemia was similar in both groups. Our study also included 25 patients from advanced CKD outpatient clinics. In these patients, the percentage decrease in phosphorus levels was similar to that achieved in the population on dialysis. There were no changes in calcium or PTH levels throughout the follow-up period.
Despite the fact that the technical data sheet recommends a dose of 1500 mg/day of OSF (3 tablets), in our case, the initial dose of the drug did not reach the one gram daily reaching an average increase of 200 mg (total of 1,2 g day)at the end of the study. This data informs about its efficacy and bring us to the conclusion that the total cost of treating hyperphosphataemia with OSF is lower than initially considered. In this regard, it has been published that OSF is cost-effective when compared to sevelamer over a 10-year treatment period.38
Although there was not statistical significance in parameters related to anemia and iron kinetics throughout the follow-up, it was observed an upward trend in hemoglobin and transferrin saturation index. Our study was not designed to analyze the causes of these changes, so these variations cannot be attributed exclusively to OSF. Some patients included in the analysis had their dose of erythropoiesis-stimulating agents or iron (both oral and intravenous) modified; in addition, 2 patients required transfusion of blood products. Other studies have analyzed the role that the administration of an iron-containing phosphorus binder may have in the control of anemia and ferrokinetic parameters. Floege et al.16 also found an upward trend in all these parameters, which did not reach statistical significance either. However, Kendrick et al.18 did find a significant increase in hemoglobin, ferritin, and transferrin saturation index in their large series of HD patients, with a decrease in the need for erythropoiesis-stimulating agents and intravenous iron. It would not be surprising that part of the iron of the OSF molecule could be absorbed at the intestinal level and have an effect in the parameters described.
Regarding adverse effects, it should be noted that 10 patients (11.7% of the total) abandoned the treatment due to some adverse effect associated with the drug, mainly the presence of diarrhea. The dropout rate with OSF in our study is lower than that published by Floege et al.16 and similar to that described in previous studies with sevelamer or lanthanum.39,40 All patients had black stools, which is explained by the elimination of iron contained in the tablets in the stool. The consistency of the stool was, in general, softer, especially the first weeks of treatment. This effect, which could be considered adverse, could have an added beneficial effect in PD patients, who frequently need to use laxatives to maintain adequate bowel rhythm and avoid peritoneal catheter displacement or drainage problems. In this regard, a Spanish multicenter study is underway that will assess the efficacy of OSF in controlling phosphorus in PD patients and will also evaluate the intestinal rhythm and the possible reduction in the need for laxatives.
Among the limitations of the present work, it should be noted that it is a clinical practice study and there is not a control group to compare the efficacy of the drug. However, it has the strength of being a multicenter study, with physicians from various fields of nephrology and various hospitals, who follow the usual clinical practice. On the other hand, the incorporation of PD patients and especially the advanced CKD patients from the outpatient clinics provides information on groups of patients with little or no information available, up to now.
In conclusion, our study demonstrates the efficacy of OSF in a sample of dialysis patients, both HD and PD, and also in patients with advanced CKD. The decrease of more than 25% in serum phosphorus levels, at a dose significantly lower than that recommended in the technical data sheet, indicate the high potential of this drug. Adverse effects, especially diarrhea, may limit the positive effect of this drug in about 10% of patients.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Sanchez-Alvarez JE, Astudillo Cortés E, Seras Mozas M, García Castro R, Hidalgo Ordoñez CM, Andrade López AC, et al. Eficacia y seguridad de oxihidróxido sucroférrico en el tratamiento de la hiperfosforemia en la enfermedad renal crónica en Asturias. Estudio FOSFASTUR. Nefrologia. 2021;41:45–52.