El magnesio es el catión extracelular más abundante en el cuerpo humano y el segundo más abundante intracelular después del potasio. Es esencial para la transferencia, almacenamiento y utilización de la energía como regulador y catalizador de más de 300 sistemas enzimáticos. La hipomagnesemia puede producir una variedad de anormalidades metabólicas y consecuencias clínicas. Puede resultar del desequilibrio entre la absorción intestinal y la excreción renal. La principal consecuencia relacionada directamente con la hipomagnesemia son las arritmias cardiovasculares por hipopotasemia secundaria, y si no se reconoce y trata puede ser fatal. En este artículo revisamos las hipomagnesemias haciendo hincapié en los mecanismos moleculares responsables de la homeostasis del magnesio, diagnóstico diferencial y tratamiento, a propósito de la descripción de las manifestaciones clínicas y bioquímicas y el defecto genético en una familia afectada de síndrome de Gitelman.
INTRODUCTION
Magnesium is the fourth most abundant cation in the body and the second most abundant cation in the intracellular compartment. It is essential for the function of many enzymes, including enzymes which are associated with the transfer of phosphate groups, all the reactions which require ATP, all the steps involved in the replication and transcription of DNA, and the translation of mRNA. It is estimated that the total amount of magnesium in the body is 1,000mmol or 22.66g; 99% of this total amount of magnesium is located in the intracellular compartment, 60% in the bones, 20% in the muscles and another 20% in other tissues. The translocation of magnesium from the intracellular to the extracellular compartment occurs slowly over a period of several weeks. Only 1% of the total magnesium in the body is found in the extracellular compartment. 60% of this extracellular total is free or ionized magnesium, 10% is bound to citrate, phosphate, and oxalate salts and other anions, with which it forms compounds, and 30% is bound to proteins. The concentration of magnesium in plasma is maintained within a narrow range of 1.7 to 2.2mg/dl (0.75-0.95mmol/l or 1.5-1.9mEq/l). The homeostasis of magnesium depends on a balance between its intestinal absorption and renal excretion. Hypomagnesaemia is defined as a plasma concentration of magnesium which is lower than 1.7mg/dl (< 0.75mmol/l or < 1.5mEq/l).
PHYSIOLOGY OF MAGNESIUM HOMEOSTASIS
In an average diet, 360mg (15mmol) of elemental magnesium are ingested. The daily requirement of elemental magnesium is 0.15 to 0.20mmol/kg. Sources which are rich in magnesium include cereals, seeds, walnuts, pulses, chocolate, green vegetables, and certain kinds of meat and seafood. Normally, only 50% of the magnesium in our diet is absorbed in the gastrointestinal tract, primarily in the proximal jejunum and the ileum. About 40mg/day of magnesium are also secreted into the intestine and, only 20mg are reabsorbed in the colon and rectum.
The absorption of magnesium occurs in the ileum by means of two processes:
The first is an active and saturable process, which is the principal route for the transport of magnesium. It occurs across the TRPM61-3 magnesium (transient receptor potential melastatin) channel. A second mechanism, which is passive and non-saturable, occurs via the paracellular route.
Magnesium is essential for the transfer, storage and use of energy, and it regulates and catalyzes over 300 enzyme systems. Hypomagnesaemia can therefore lead to various metabolic abnormalities and clinical consequences, which are caused by an imbalance between the gastrointestinal absorption and renal excretion of magnesium. The principal manifestation of hypomagnesaemia is heart arrhythmias, which, unless diagnosed and treated, can be fatal.
80% of the magnesium found in plasma, 95% of which is reabsorbed by nephrons, is filtered by the glomeruli. Unlike other ions, the tubular absorption of magnesium occurs primarily in the thick ascending limb of Henle¿s loop, and it accounts for 60 to 70% of the total amount which is filtered. The proximal tubule absorbs only 15-25% of the magnesium which is filtered; in turn, the distal tubule absorbs 5-10% of the magnesium which is filtered, but it is regarded as the final control point in the regulation of magnesium.1 In the thick ascending limb of Henle¿s loop magnesium is passively reabsorbed together with calcium via the paracellular pathway, which is composed of tight intercellular bonds. The momentum underlying this reabsorption is the electric gradient generated as a result of the reabsorption of sodium by the co-transporter Na+/K+/2Cl-(NKCC2). Paracelin-1, also known as claudin-16, which has been identified as the protein which forms these tight intercellular bonds.4-6
In the distal tubule, magnesium is reabsorbed by means of an active mechanism, involving the TRPM6 magnesium channel.7 The mechanism for transporting magnesium in the basolateral membrane of the cells in the thick ascending limb of Henle¿s loop and the distal tubule is unknown. The transport of magnesium across this membrane must be against an electrochemical gradient. Most studies suggest there is a sodium-dependent exchange mechanism, which would be favoured by low concentrations of intracellular sodium generated by the Na+/K+-ATPase pump.8
FACTORS WHICH INFLUENCE THE EXCRETION OF MAGNESIUM BY THE KIDNEY
1. The plasma concentration of magnesium is the principal regulator of magnesium in the kidney. Hypermagnesaemia inhibits the reabsorption of magnesium in the thick ascending limb of Henle¿s loop, while hypomagnesaemia stimulates it. The plasma concentration of calcium has a similar effect. Hypermagnesaemia and hypercalcaemia inhibit magnesium reabsorption by activating the calcium sensor-receptor in the cells in the thick ascending limb of Henle¿s loop and the distal tubule. When magnesium or calcium activate the receptor, this stimulates the formation of an arachidonic acid derivate, which irreversibly inhibits potassium channels in the thick ascending limb of Henle¿s loop. The secretion of potassium has two functions: firstly, it provides potassium for the reabsorption of sodium and chlorine by the cotransporter NKCC2 and, secondly, it intervenes in the production of the electric gradient required for the passive reabsorption of magnesium and calcium.9 Consequently, the inhibition of potassium channels in the thick ascending limb of Henle¿s loop would reduce sodium transport and the passive reabsorption of magnesium and calcium.10,11
2. Extracellular fluid volume also influences magnesium excretion. An increase in volume inhibits magnesium reabsorption in the thick ascending limb of Henle¿s loop, probably as a result of an increase in the sodium load, and, consequently, a decrease in the electric gradient, which promotes the paracellular transport of magnesium.
3. Changes in the glomerular filtration rate can also affect renal magnesium excretion. When the glomerular filtration rate decreases and, as a result, the amount of magnesium which is filtered, the reabsorption of magnesium is reduced.
4. Phosphate depletion lowers the reabsorption of magnesium by an unknown mechanism.
5. Chronic metabolic acidosis causes renal loss of magnesium, while chronic metabolic alkalosis has the opposite effect. Chronic metabolic acidosis reduces the expression of the TRPM6 magnesium channel in the distal tubule, decreasing magnesium reabsorption in this part of the glomerulus. Chronic metabolic alkalosis increases the expression of this channel, which has the opposite effect.
Several hormones, including 1.25(OH)2 vitamine D, parathormone, calcitonin, glucagon, aldosterone, anti-diuretic hormone, insulin, prostaglandin E2 and catecholamines, stimulate magnesium reabsorption in the thick ascending limb of Henle¿s loop and the distal tubule. The mechanism is unknown, but in many cases it is believed to be related to an increase in intracellular cAMP. Furthermore, recent studies show that 1.25(OH)2 vitamin D3 can also produce an increase in the expression of paracelin-1 by activating the PPAR transcription factor and subsequently bonding with the specific PPRE response element in the promotor region of the hPCLN-1 gene.
CAUSES OF HYPOMAGNESAEMIA
Hypomagnesaemia can occur as a result of four physiopathological mechanisms:
A decrease in food intake
Decreased food intake rarely causes magnesium deficiency, as most foods contain significant quantities of this element and the kidney is able to adapt and store magnesium very efficiently. However, hypomagnesaemia can occur in three patient groups: undernourished patients, alcoholics and patients who are administered total parentheral nutrition for prolonged periods of time.
Redistribution
The translocation of magnesium from the extracellular to the intracellular environment is a common cause of hypomagnesaemia. This can occur in so-called hungry bone syndrome, in which magnesium is deposited in the bones. This syndrome occurs in patients with hyperparathyroidism after they have undergone a parathyroidectomy or in patients with hyperthyroidism after a thyroidectomy.
Hypomagnesaemia can also develop as a result of hyperinsulinaemia during the treatment of diabetic ketoacidosis, in refeeding syndrome or during the intravenous administration of dextrose.
Gastrointestinal loss
Changes in magnesium absorption in the intestine can occur as a result of diarrhoea due to any cause or as a result of surgical resection of the intestine. Patients with ileostomies can develop hypomagnesaemia because there is a certain degree of magnesium reabsorption in the colon.
Hypomagnesaemia with secondary hypocalcaemia (HSH) is the result of an autosomal recessive mutation which is characterized by severe hypomagnesaemia associated with hypocalcaemia. The physiopathology of hypomagnesaemia in this disease is linked to a defect in magnesium reabsorption in the intestine and in the distal tubule. Recently, mutations in the TRPM6 gene, which expresses the TRPM6 magnesium channel, have been identified as the underlying genetic cause.
Renal Loss
Various hereditary tubular defects are responsible for the urinary loss of magnesium. Gitelman¿s syndrome is an autosomal recessive disorder caused by mutations of the SCL12A3 gene, which expresses the NaCl cotransporter (NCCT) in the distal tubule. This syndrome is characterized by hypokalaemia, hypomagnesaemia and hypocalciuria associated with metabolic alkalosis. Hypomagnesaemia is present in the majority of patients with Gitelman¿s syndrome and in the past it was assumed to be related to a defect in the NCCT cotransporter, but the exact mechanism was unknown. Recently, some studies suggest that the loss of magnesium could be due to decreased expression of the TRPM6 magnesium channel in the distal tubule.12-16
Out of the five variants of Bartter¿s syndrome, only classic or type III Bartter¿s syndrome is linked to hypomagnesaemia. This variant of Bartter¿s syndrome is caused by mutations in the CLCNKB gene, which expresses the CLC-Kb chlorine channel located in the basolateral membrane of the thick ascending limb of Henle¿s loop and the distal tubule. This channel measures the flow of chlorine into interstices. The mechanism underlying hypomagnesaemia in this syndrome is unknown.
CLINICAL MANIFESTATIONS OF HYPOMAGNESAEMIA
Most patients with hypomagnesaemia present no symptoms. The symptoms of hypomagnesaemia do not appear until the plasma concentration of magnesium falls to values below 1.2mg/dl. Furthermore, hypomagnesaemia is accompanied by other electrolyte changes, such us hypokalaemia and hypocalcaemia, which make it difficult to distinguish clinical manifestations17-20 which are specifically related to magnesium deficiency. The clinical manifestations of Gitelman¿s syndrome are very heterogenous, the symptoms being as subtle as dizziness or vertigo, muscle weakness, cramps and muscular pain, joint pain, fatigue or tiredness.21-25.
Hypokalaemia20 is a common finding in patients with hypomagnesaemia, and occurs in 40-60% of cases. This is partly due to the underlying disease that causes losses of both magnesium and potassium, which is what happens, for example, in patients who take diuretics or have diarrhoea. However, the chief mechanism underlying hypokalaemia caused by hypomagnesaemia is in fact related to the intrinsic biophysical properties of the ROMK1 channels26 ,which mediate potassium secretion in the thick ascending limb of Henle¿s loop. ROMK1 channels are internal potassium rectifiers, which means they show greater conductance for the potassium which flows into the cell than for potassium flowing out of the cell. The mechanism underlying this preferential conductance into the cell is the result of the bonding and subsequent blocking of the conductance of potassium out of the cell by intracellular magnesium and polyamines. Areduction in intracellular magnesium as a result of magnesium deficiency would induce a decrease in internal rectification and, consequently, an increase in the conductance of potassium out of the cell, with a corresponding loss of potassium and hypokalaemia.
Whatever the case, hypokalaemia induced by hypomagnesaemia is characterized by being refractory to treatment with potassium supplements and can only be resolved by correcting the magnesium deficiency.
Hypomagnesaemia can also induce hypocalcaemia. This generally occurs when the hypomagnesaemia is severe (< 1.2mg/dl). The mechanism is multiple. There is a decrease in the release of parathormone. The details of the mechanism are unknown, but it is thought that magnesium might increase the activity of the alpha subunit of the G protein related to CaSR. Hypomagnesaemia can also cause resistance to the action of parathormone on bone tissue. It seems that magnesium deficiency interferes with the production of cAMP, which is the intracellular mediator for parathormone.
GENETIC STUDY
The primary defect in this pathology is a mutation that inactivates the SLC12A3 gene located on chromosome 16, which codes for the thiazide-sensitive Na-Cl transporter of the distal convoluted tubule.27-32 The intron 9 +1G>T mutation is characteristic of gypsy populations. In the case in question, the analysis was performed on the patient and her siblings.
Case report
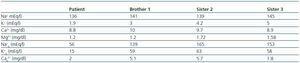
We present the case of a formerly healthy 8-year-old gypsy girl who was referred to the hospital suffering from continuous and diffuse abdominal pain of recent onset associated with vomiting, fever and dysuria (table 1).
The genetic study demonstrated that the patient was homozygotic for the intron 9 +1G>T mutation of the SLC12A3 gene.33-39
A genetic study performed on the family obtained the following results:
- Brother 1: homozygote for the mutation.
- Sister 2: normal homozygote, non carrier.
- Sister 3: heterozygote, carrier of the mutation.
DIAGNOSIS OF HYPOMAGNESAEMIA
The simplest way to evaluate magnesium deficiency is to measure the plasma concentration of magnesium.40-43 With regard to the rest, there are two important points to consider. 30% of magnesium is bound to albumin; therefore hypoalbuminaemia can trigger ¿pseudohypomagnesaemia¿. On the other hand, most of the magnesium in the body is located in the intracellular compartment. Therefore, a person can have normal plasma levels of magnesium and still present an intracellular magnesium deficiency and show signs of hypomagnesaemia;44-47 this is known as functional magnesium deficiency. Unbound ionized magnesium in plasma is the physiologically active form and, in addition, the form which best reflects intracellular magnesium reserves. Unfortunately, in current clinical practice there is no laboratory assay that can measure plasma concentrations of free magnesium.
A way of evaluating functional magnesium deficiency in patients with normal plasma concentrations of magnesium, but in whom there is reason to suspect a magnesium deficiency, is to measure plasma concentrations of magnesium after magnesium loading. Firstly, baseline magnesium excretion is measured in 24 hours urine. After that an infusion of 7.5g of magnesium sulphate is administered after 8 hours and then magnesium excretion is measured after 24 hours if the patient excretes < 70% of the magnesium load plus their baseline magnesium excretion, this is regarded as functional magnesium deficiency.
If the cause is not evident, the distinction between renal and gastrointestinal magnesium loss can be made by measuring the amount of magnesium in a 24 hours urine sample or by calculating the magnesium excretion fraction in a urine sample obtained at random. An EFMg higher than 3% or over 1mmol (24mg) of magnesium in 24 hours urine would indicate renal loss of magnesium.
TREATMENT OF HYPOMAGNESAEMIA
In general, patients with hypomagnesaemia must follow a diet which is rich in magnesium and the cause of hypomagnesaemia must be treated, whenever posible.48
If the patient has no symptoms or the hypomagnesaemia is not severe (plasma magnesium level > 1mg/dl), oral administration is the route of choice, preferably using prolonged-release preparations, such as magnesium chloride or lactate. In cases where there are symptoms or when the concentration of magnesium is < 1mg/dl, intravenous administration is the preferred route. The preparation of choice is magnesium sulphate.
The plasma magnesium values must be monitored, looking for signs of toxicity, such as oliguria, impaired consciousness and absence of reflexes. Patients with renal failure should receive 50% of the dose if their serum creatinine level is higher than 2. In cases of toxicity, the antidote is intravenous calcium chloride or gluconate. Patients with hypomagnesaemia induced by diuretics, which for some reason cannot be interrupted, may benefit from the use of amiloride, which can reduce the excretion of magnesium in the distal tubule. Amiloride appears to cause hyperpolarization of the cell membrane, which would encourage the production of the transmembrane potential required for magnesium reabsorption.
Table 1.