Post transplant lymphoproliferative disorders (PTLD) are heterogeneous lymphoid proliferations in recipients of solid organs which seem to be related to Epstein Barr Virus (EBV). The use of antilymphocyte antibodies, EBV seronegativity in the recipient,acute rejection and CMV infection have been identified as classical risk factors.
Material y methodsWe have studied in a retrospective observational study, the incidence of PTLD in a period of 22 years, its relationship with EBV, presence of classical risk factors and outcome in 21546 simple adult renal transplant recipients from cadaveric and living donors, transplanted in 21 hospitals from 1990 to 2009.
ResultsA total of 275 recipients developed PTLD (1,2%),195 males (70,9%), 80 females (29,1%) aged 59.2 (p25 44.7 p75 68)years. Two hundred forty-five (89.0%) were 1st transplant recipients and 269 (97,8%) from cadaveric donors. EBV in the tissue was reported in 94 out of the 155 studied recipients (60.6%) and 86.0% of the proliferations were due to B lymphocytes. PTLD median appearance after transplant were 42.months (p25, 75, 12, 77, 5). One hundred eighty-eight recipients out of 275 patients (68.3%) had any classical risk factor and the use of antilymphocyte antibodies was the most frequent.
During the follow-up, 172 patients died (62,5%) and 103 (37,5%) had a complete remission. The main cause of death was PTLD progression (n = 91, 52,9%), followed by sepsis (n = 24, 13,9%). The follow-up period post-transplant of the recipients was between 3 and 22 years.
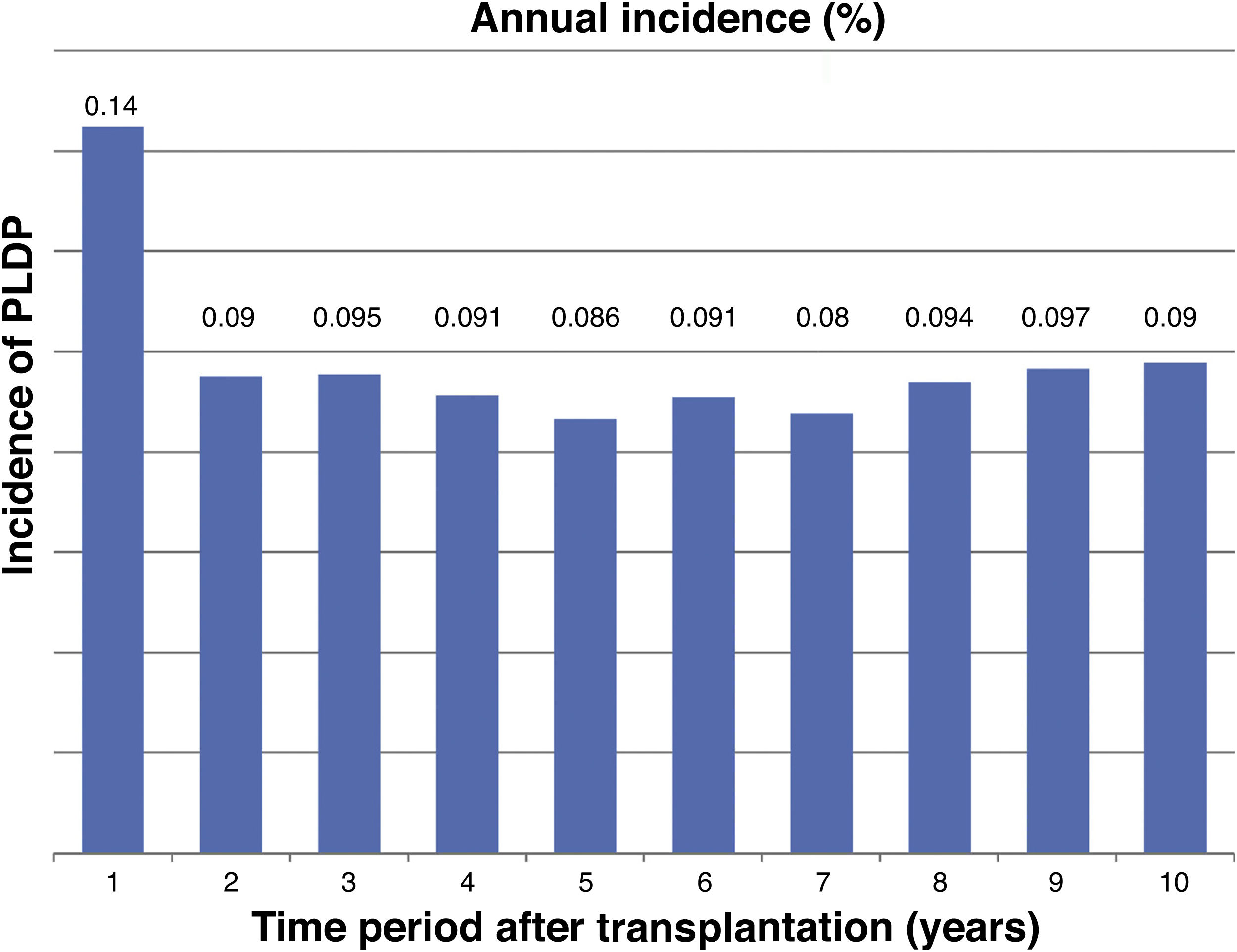
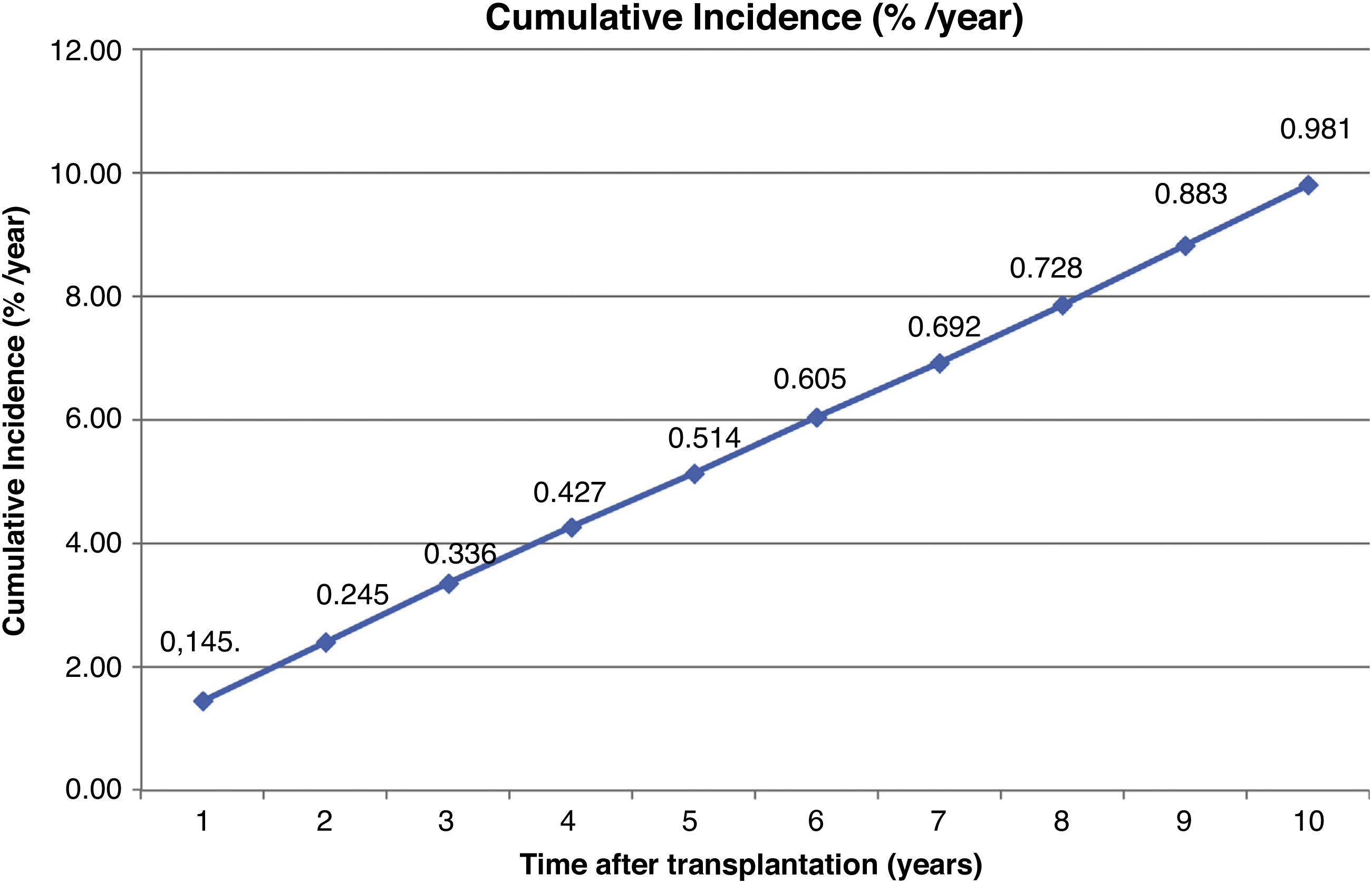
The incidence was 0,14% during the first year post-trasplant and 0.98% the cumulative incidence at 10 years.
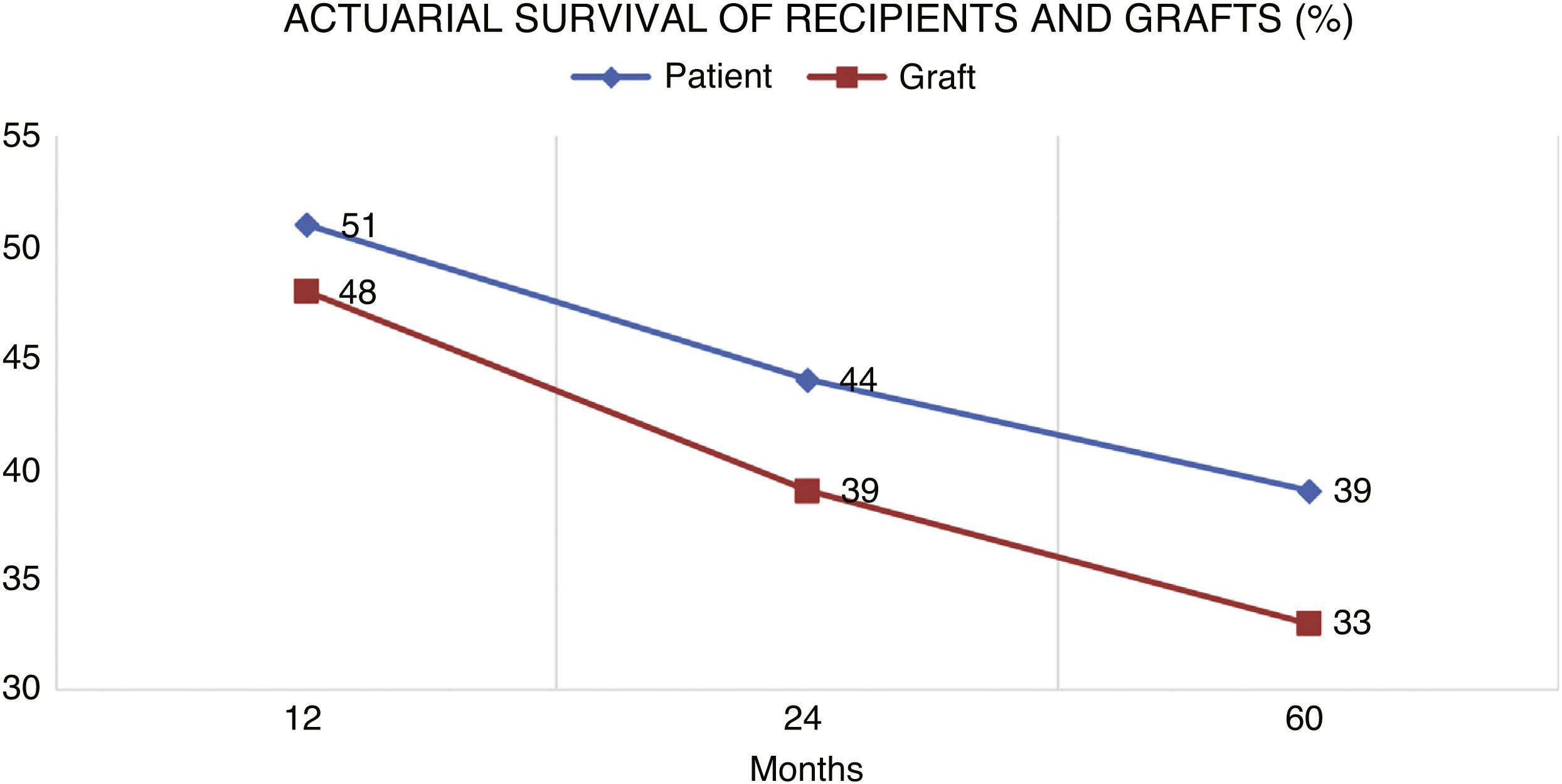
Patient survival after diagnosis was 51%, 44% and 39% after 1, 2 and 5 years, respectively. Finally, overall graft survival was 48%, 39% and 33% at the same periods.
ConclusionPTLD has a low incidence in renal transplant recipients. Most of the proliferations are due to B lymphocytes and seem to have a close relationship with EBV. PTLD can develop in the absence of classical risk factors. The prognosis is poor, mainly due to PTLD progression, but the survivors can even maintain their grafts.
La enfermedad linfoproliferativa difusa post – trasplante (ELPD) es un grupo heterogéneo de enfermedades que se caracteriza por una proliferación de linfocitos después de un trasplante de órgano sólido y que presenta un espectro que comprende desde hiperplasias a agresivos linfomas.
Material y métodosHemos evaluado, en un estudio observacional multicéntrico retrospectivo que incluye 21.546 receptores adultos de trasplante renal simple trasplantados en España de 1990 al 2009, la incidencia de ELPD durante un periodo de 22años,su relación con el Virus Epstein Barr(VEB), los factores de riesgo clásico y su pronóstico.
ResultadosUn total de 275 receptores desarrollaron ELPD durante el seguimiento (1,2%). 195 varones (70,9%), 80 mujeres (29,1%), con una mediana de edad al diagnostico de 59.2 (p25 44.7 p75 68) años. Doscientos cuarenta y cinco (89.0%) eran primeros trasplantes y 269 (97,8%) de donante cadáver. Se objetivo VEB en el tejido proliferativo en 94 de 155 casos estudiados (60.6%) y el 86.0% de las proliferaciones eran linfocitos B. La mediana del tiempo de desarrollo después del trasplante fue de 42. meses (p25, 75, 12, 77, 5). Un total de 188 receptores de 275 (68.3%) tenían algún factor de riesgo clásico.
La incidencia anual fue 0,14% el primer año y 0.98 la acumulada en 10 años post-trasplante.
El periodo de seguimiento post-trasplante de los receptores fue de 3 a 22 años.
Durante el seguimiento 172 pacientes murieron (62,5%) y 103 (37,5%) tuvieron remisión completa. La causa de muerte más frecuente fue la progresión (n = 91, 52,9%), seguida de sepsis (n = 24, 13,9%).
La supervivencia del paciente después del diagnóstico fue del 51% al año, del 44% al 2º año y 39% al 5º año. La supervivencia del injerto fue de 48,39 y 33%.
ConclusiónEste estudio muestra una baja incidencia de ELPD en receptores de trasplante renal en un periodo de 22 años. La mayoría de las proliferaciones se asocian a Linfocitos B y presentan una importante relación con VEB. La entidad puede desarrollarse en ausencia de factores de riesgo clásicos y su incidencia es mayor en el 1º año post-trasplante, presentando un mal pronóstico principalmente en los primeros meses de la enfermedad que condiciona una mala supervivencia del paciente que si sobrevive puede mantener su injerto.
Post-transplant diffuse lymphoproliferative disease (PLPD) is a heterogeneous group of disorders characterized by a proliferation of lymphocytes after solid transplantation, with a spectrum ranging from hyperplasia to aggressive non-Hodgkin's lymphomas.1,2
The incidence of PLPD is highly variable and depends on the transplanted organ; renal transplantation has the lowest incidence.1,3 However, because renal transplantation is the most common transplant performed each year, most PLDP is diagnosed in renal transplant recipients.
This entity is more prevalent in solid organ transplant recipients than in the general population and its mortality rates is also higher.4 These proliferations are mostly of B lymphocyte origin5,6 and the Epstein-Barr virus (EBV) has long been considered to be involved in their pathogenesis.6–8 The development of this disease is considered to be an iatrogenic complication of immunosuppressive treatment associated with solid organ transplantation, which leads to reduced immune control of this virus by T-cells, resulting in the proliferation of EBV-infected B-cells.6
Classically there have been described several risk factors in the development of this entity9–12; such factor are EBV seronegative recipients who receive grafts from seropositive donors,4,7,9,12 the burden of immunosuppression especially with the use of antilymphocyte antibodies,7,11–13 acute rejection12,14 and cytomegalovirus infection.10,11,15
We have evaluated the incidence of PLDP over a 20-year period, in a retrospective multicenter observational study that included a cohort of 21,546 adult single kidney transplant recipients transplanted in Spain from 1990 to 2009. The study collects demographic and clinical data and different risk factors involved in the development and prognosis of this entity.
MethodsThe present study is a multicenter, nationwide, retrospective, observational, multicenter study that included 21 Spanish tertiary hospitals.
The study population included 21,546 adult recipients who received a single kidney transplant from a cadaveric or living donor from January 1, 1990 to December 31, 2009. The study period was 22 years, from January 1, 1990 to December 31, 2012.
Inclusion criteria were: recipients older than 18 years at the time of transplantation and with a functioning graft at the time of PLDP diagnosis. The diagnosis of PLDP was made by histopathological analysis when biopsy was available or based on strong clinical suspicion when biopsy was not possible. The histological study was performed by the pathologist of the corresponding center.
Data from the 21 participating hospitals were collected by means of a designed electronic questionnaire, which included the different study variables. Time to development of PLDP was defined as the period in months from organ transplantation to the date of PLDP diagnosis. Follow-up ended when patient died or the end of the study, December 31, 2012.
The analysis included sociodemographic variables such as age and gender, clinical data such as date of transplantation, type of donor (cadaver or living), number of transplants performed on the patient, immunosuppression at the time of PLDP diagnosis, date of diagnosis, diagnostic method, presence of EBV in the proliferative tissue, type of proliferation (B or T), classical risk factors and evolution (complete remission, graft loss, death, loss to follow-up).
Immunosuppression treatments included the use of the following agents, (alone or in combination): tacrolimus (TAC), cyclosporine A (CsA), azathioprine, mycophenolate mofetil (MMF), everolimus, sirolimus, steroids and antibodies (ATG, ATGAM, OKT3, anti-CD 25, thymoglobulin).
The following risk factors were evaluated: seronegative EBV recipients, cytomegalovirus infection, acute rejection, and treatment with mono- or polyclonal antibodies at induction or in the treatment of acute rejection.
The incidence of PLDP was calculated as the number of cases observed over the total population at risk and the annual and 10-year cumulative incidence was assessed.
Statistical analysisStatistical analysis was performed with SPSS 19.0 software for Windows (SPSS Inc., Chicago, IL, USA). Patient demographic characteristics were expressed as percentages for quantitative variables. Independent qualitative variables were analyzed using contingency tables with the chi-square statistical method.
Means with standard deviation were determined for quantitative variables with normal distribution. When the variables did not follow a normal distribution, the median with interquartile range was used. Survival of both recipients with PLDP and the grafts was analyzed using Kaplan–Meier survival curves.
ResultsDuring the study period there were a total of 21,546 renal transplants performed and 275 recipients developed PLDP (1.2%).
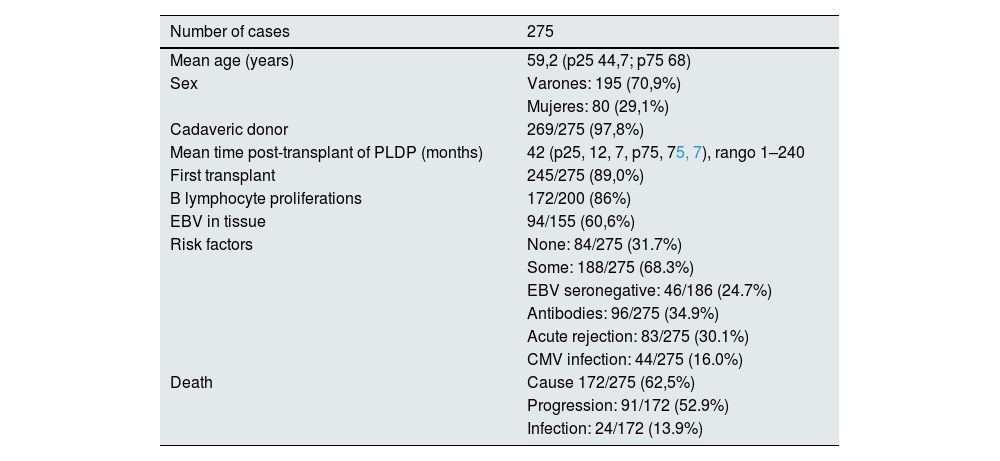
Table 1 shows the sex and mean age at the time of diagnosis of the disease, the time after transplant of onset of the disease, the percentages of first transplants, type of donor, B lineage proliferation and the presence of EBV in the proliferative tissue. The percentages of patients with some classical risk factor in total and of the different factors individually are also expressed, as well as the percentage of deaths and the cause of these deaths.
Clinical data of recipients who developed diffuse post-transplant diffuse lymphoproliferative disease.
| Number of cases | 275 |
|---|---|
| Mean age (years) | 59,2 (p25 44,7; p75 68) |
| Sex | Varones: 195 (70,9%) |
| Mujeres: 80 (29,1%) | |
| Cadaveric donor | 269/275 (97,8%) |
| Mean time post-transplant of PLDP (months) | 42 (p25, 12, 7, p75, 75, 7), rango 1–240 |
| First transplant | 245/275 (89,0%) |
| B lymphocyte proliferations | 172/200 (86%) |
| EBV in tissue | 94/155 (60,6%) |
| Risk factors | None: 84/275 (31.7%) |
| Some: 188/275 (68.3%) | |
| EBV seronegative: 46/186 (24.7%) | |
| Antibodies: 96/275 (34.9%) | |
| Acute rejection: 83/275 (30.1%) | |
| CMV infection: 44/275 (16.0%) | |
| Death | Cause 172/275 (62,5%) |
| Progression: 91/172 (52.9%) | |
| Infection: 24/172 (13.9%) |
CMV, cytomegalovirus; PLDP, diffuse lymphoproliferative disease; EBV, Epstein-Barr virus.
A total of 182 of the 275 cases were transplanted between 1990 and 1999 and at the time of diagnosis a 77.1% of cases received CsA, MMF was administered to 24.7% and FK to only 17.1% of cases. The remaining 93 cases had been transplanted during the period 2000–2009, with an evident change in immunosuppression, TAC was used in 78.1% of the cases, 76.7% received MMF and only 16.4% were treated with CsA.
The diagnosis of PLDP was made by histological analysis in 260/275 recipients (94.5%), in 16 of them at necropsy and in the remaing 15 patients (5.5%) the diagnosis was made on clinical data and complementary examinations.
The annual incidence is shown in Fig. 1 and the cumulative incidence at 10 years post-transplantation in Fig. 2.
Shown in Fig. 3 is the patient survival after diagnosis was 51% at 1 year, 44% at 2 years and 39% at 5 years and the graft survival 48%, 39% and 33%, respectively.
The minimum post-transplant follow-up of the recipients was 3 years and the maximum 22 years.
DiscussionWe have conducted a longitudinal multicenter study in Spain over a long period of time, from 1990 until 2012, to evaluate the incidence and prognosis of PLDP. This study has allowed to improve knowledge of the real impact of this potentially fatal complication. The information available was based on previous single-center studies with rather low number of cases and therefore a limited statistical evidence.16 In the present study we selected only adult recipients, as the epidemiology of PLDP in pediatric patients is not the same as in adult population.17,18
The overall incidence observed in our study was 1.2%, this is within the classic range for renal transplantation of 1–3%,3,12,19,20 while the 10-year cumulative the incidence was 0.98%, while Quinlan et al. report 1.4%,21 Opelz and Döhler (1.6%)22 and Caillard et al. 2.1%.7
The incidence seems to decrease with the time elapsed after transplantation. Thus, a study published by Caillard et al. shows a reduction in incidence in the period from 2002 to 2005 compared to the period from 1998 to 2001.7 A national study from Sweden shows a decrease in incidence during the last decade, but only among non-renal recipients.23 The authors consider that the more rational use of antibodies and the use of new immunosuppressive drugs are responsible for this change. However, other studies show no significant differences in the incidence of PLDP in the different time periods.10,22 In our study we recorded a lower number of cases in the second decade, but it must consider that the follow-up of this period of time has been shorter.
The different immunosuppressive agents and load of immunosuppression have been directly implicated in the development of PLDP and have been extensively studied by others.13,16,23–25 In general it is being considered that immunosuppression is a key factor in the development of PLDP in renal transplant recipients.24 The fact that patients with graft failure that need to restart dialysis have a lower risk of developing the disease confirms this hypothesis.25,26 It is worth noting the trend towards a change in calcineurin inhibitors in the last decade, a fact that was endorsed in our study and which could have changed the incidence of PLDP. The most commonly used immunosuppressants in the second decade were TAC and MMF, which according to several studies,7,12,27 are associated with a lower risk of developing the disease; but, the relationship between the use of FK and PLDP remains controversial,28 although its impact seems to be less than that of CsA.29 It should be noted that although CsA is a less potent immunosuppressant than TAC, the more frequent concomitant use of mono- or polyclonal antibodies for induction with higher cumulative dose, could explain the increased incidence of PLDP in that period.7,23
One third of the cases analyzed in our study did not present an associated classic risk factor (Table 1). This observation highlights the lack of knowledge about all factors that could influence the development of PLDP, as well as the heterogeneity of this type of disease, which makes prediction strategies difficult. Along these lines, different studies have evaluated and proposed other risk factors, such as recipient age, ethnicity, HLA incompatibility between donor and recipient, serum creatinine, LDH levels and the presence pre-transplant tumors.7,9,12,30
There is controversy as to whether there are 2 different types of entities within PLDP depending on the time of their appearance.5,9,21,24 Thus, if the study is long enough to represent a cumulative curve of incidence, it is usually observed a bimodal curve.7,9,21,25 The first elevation would correspond to proliferations during the first year, related to EBV infection and responds well to a decrease in immunosuppression.21,25,31–33
The second elevation appears long after transplantation, even decades, and it is rarely associated with EBV, with a poor evolution and low sensitivity to a reduction in immunosuppression.5,9,21,25,34 Because both groups have a different origin, as well as evolution, their development could be influenced differently by risk factors, which should be studied and treated differently. Quinlan et al.21 highlights the existence of the 2 entities and identify different risk factors for each one. In our study we observed a higher incidence in the first year, as also observed in other studies,7,12,13,21,22,26,35 and a stabilization during the rest of the follow-up, with no evidence of a bimodal curve, perhaps because the cumulative incidence was discontinued at 10 years.
The median time of onset of PLDP in the post-transplant period in the different series is very variable due to the great dispersion of the cases over time, as the period of time studied is very long. In our study, the median post-transplant period elapsed before the onset of the disease was 42 months, and with cases of very late onset, up to 240 months post-transplantation. Other series present longer median time periods, such as Morton et al. of 74 months, with 3 cases that developed the disease more than 20 years post-transplantation,35 or Caillard et al. of 89 months in the French registry, with a range of one to 397 months.31 These very long periods are produced by compensating a higher early incidence with the inclusion of very late cases, as the series mentioned have a very long post-transplant follow-up period. At the other extreme, we found shorter median time to onset, as in the American registry, of only 12 months, when analyzing the series over a much shorter period of time.12 In conclusion, the median time of onset of the disease seems to be proportional to the follow-up time of the series.
The pathogenesis of PLDP is associated to EBV.6–8,24 This association is related to an EBV-specific immune response resulting in uncontrolled reactivation of the virus or primary infection.6 The etiology of EBV-negative PLDP appears to be due to age-related loss of immune surveillance.21 The PLDP-EBV relationship seems clear; our study showed the presence of the virus in 60.6% of the cases in which it was analyzed in the proliferative tissue (Table 1). In their study, Morton et al. detected EBV in 68% of the cases studied in their series,35 values that are similar to ours, which supports our data.
The prognosis of patients with PLDP is poor, much worse than in recipients who do not develop the disease.12,16,31,35–37 In our study, the actuarial survival of the recipient was low (39% at 5 years) (Fig. 3), similar to the range presented in other studies, such as that of Faull et al. who report the same 5-year survival36 or that of Opelz and Döhler,22 perhaps also marked by the presence of very old cases without access to current treatments.38,39
The most extensive experience in PLDP in adult renal transplant recipients has been collected in the French registry, which includes 500 patients diagnosed between 1998 and 2007, with actuarial survival of 53 and 45% at 5 and 10 years after diagnosis, respectively.31 Data obtained from the American database show a somewhat better survival of 64% at 5 years, but significantly lower than that of recipients who do not develop PLDP.12,16
The development of PLDP significantly decreases patient survival, mainly during the first year,16,22,36 since in that period the immunosuppression burden is higher and the occurrence of opportunistic infections more frequent,13,16,23–25 but graft survival is good if those patients that survive, so most of the patients who did not die maintained their grafts (Fig. 3).
Multivariate analysis of the French registry revealed 5 variables at diagnosis that were independently associated with inferior survival: older age (>55 years), serum creatinine >1.5 mg/dL, elevated LDH, disease location, and monomorphic or T-cell histology.31
The main limitation of our study is associated with its retrospective nature, as well as the shortcomings inherent to large databases, such as differences in clinical practice and the lack of some data especially in relation to EBV serostatus and its determination in proliferative tissue, a practice that is routinely performed in recent years but not in the earlier years. The collection of risk factors in the transplanted population that did not develop the disease would have been key for comparative purposes in order to draw conclusions.
The strength of our study is the large number of patients included, all adults, recipients of single renal transplantation and with an extensive follow-up period. In addition, the diagnosis of the disease was made in more than 94% of the series on histological grounds, which gives reliability to the diagnosis of the cases.
In conclusion, this nationwide study shows a low incidence of PLDP in renal transplant recipients during a 20-year period. Most proliferations are associated with B lymphocytes and present an important relationship with EBV. The entity may develop in the absence of classical risk factors and its incidence is higher in the first post-transplant year, presenting a poor prognosis mainly in the first months of the disease, which conditions a poor survival of the patient who, in case of survival can maintain his graft.
Conflict of interestThe authors declare that they have no conflicts of interest.
We would like to thank all the transplant nephrologists of the GREAT working group who provided the data for this study.