The optimal iron supplementation route of administration (intravenous vs. oral) in patients with chronic kidney disease (CKD) not on dialysis is a hot topic of debate. An oral preparation (liposomal iron, FeSu) has recently been developed with high bioavailability and low incidence of side effects. The objective was to evaluate the efficacy of FeSu in patients with stage 3 CKD and gastrointestinal intolerance to conventional oral iron therapy.
Material and methodsProspective observational study of patients with stable stage 3 CKD and gastrointestinal intolerance to conventional oral iron therapy. An oral 30mg/day dose of FeSu was administered for 12 months. The primary outcome measure was hemoglobin increase at 6 and 12 months. Treatment adherence and adverse effects were also evaluated.
Results37 patients aged 72.6±14.7 years and with an estimated glomerular filtration rate (eGFR) of 42±10mL/min/1.73m2 were included. 32 patients had received previous treatment with conventional oral formulations, 73% of which exhibited gastrointestinal intolerance with treatment adherence of 9.4%. After 6 months with FeSu, an increase in hemoglobin was observed versus baseline, which was sustained at 12 months (0.49±0.19 and 0.36±0.19g/dL, respectively, p<0.05), despite a significant eGFR decrease of 3.16±1.16 and 4.20±1.28mL/min/1.73m2 at 6 and 12 months, respectively. None of the patients experienced adverse reactions that required the treatment to be suspended. Adherence was 100% at both 6 and 12 months.
ConclusionsFeSu is effective in a cohort of patients with stage 3 CKD with similar characteristics to the general population of moderate CKD patients, with a low rate of adverse reactions and excellent tolerability.
La vía de suplementación óptima (intravenosa vs. oral) de hierro en pacientes con enfermedad renal crónica (ERC) no en diálisis es controvertida. Recientemente se ha desarrollado una preparación oral (hierro liposomal, FeSu) con elevada biodisponibilidad y baja incidencia de efectos secundarios. El objetivo fue evaluar la eficacia del FeSu en pacientes con ERC estadio 3 y limitación digestiva a la ferroterapia oral convencional.
Material y métodosEstudio observacional prospectivo con pacientes con ERC estadio 3 estable e intolerancia digestiva a la ferroterapia oral convencional. Se administró una dosis de FeSu de 30mg/día oral durante 12 meses. El objetivo primario fue el aumento de la hemoglobina a los 6 y 12 meses. También se evaluó la adherencia terapéutica y efectos adversos.
ResultadosSe incluyeron 37 pacientes de 72,6±14,7 años y un filtrado glomerular estimado de 42±10mL/min/1,73m2. Treinta y dos pacientes habían recibido tratamiento previo con formulaciones orales convencionales, manifestando el 73% intolerancia digestiva con una adherencia del 9,4%. Tras 6 meses con FeSu se objetivó un incremento de las cifras de hemoglobina respecto a la basal, manteniéndose a los 12 meses (0,49±0,19 y 0,36±0,19g/dL, respectivamente, p<0,05), y pese a un descenso significativo del filtrado glomerular estimado de 3,16±1,16 y 4,20±1,28mL/min/1,72 m2 a los 6 y 12 meses, respectivamente. Ningún paciente presentó reacciones adversas que obligaran a suspender el tratamiento. La adherencia fue del 100% en ambos momentos analizados.
ConclusionesEl FeSu es eficaz en una cohorte de pacientes con ERC estadio 3 de características extrapolables a la población general de pacientes con ERC moderada, con una baja tasa de reacciones adversas y excelente tolerabilidad.
Chronic kidney disease (CKD) is one of the main diseases worldwide and is associated with high morbidity and mortality, mainly at the expense of the associated cardiovascular disease.1 In Spain, CKD affects approximately a 10% of the adult population.2
CKD carries a number of potentially serious complications, anemia is one of the most frequent complications.3 Anemia has been associated to increased morbidity, mortality, progressive worsening of the quality of life of CKD patients.4 The presence of anemia is already observed in early stages of CKD (stage 3) and its prevalence increases as CKD progresses to more advanced stages.5 The study MICENAS I shows that up to 36–60% of CKD patients treated in nephrology outpatients clinics have iron deficiency and anemia.4 In many cases the iron deficiency is under-treated.4,6,7
The CKD-associated anemia is multifactorial. Besides a relative deficiency of erythropoietin, there are other factors among which iron deficiency, either functional or absolute, becomes important.8,9 Therefore, the treatment of anemia in the CKD patient is based on correcting each one of the factors causing anemia and, thereafter evaluate the initiation of agents that stimulate erythropoiesis.10–12 Iron deficiency is the most frequent cause of resistance to the action of erythropoiesis stimulating agents,13 therefore, iron supplementation is fundamental in the management of anemia of CKD patients.
Presently the objectives and strategy for iron therapy in CKD are not uniform.10,11 Another controversial point is the optimal route of iron administration. While in CKD patients on dialysis, there is a greater benefit of intravenous than oral iron therapy,14 in CKD patients not on dialysis, there is no such extensive evidence. However recent studies indicate that intravenous iron therapy may be superior in efficacy and tolerability to the classical oral route, especially with the new parenteral iron formulations.15–18
One of the main limitations of classic oral iron therapy in patients with CKD is a poor gastrointestinal (GI) tolerance and, as a consequence, low compliance.19 Recently, a preparation of ferric pyrophosphate covered by a phospholipid membrane associated with ascorbic acid (sucrosomial or liposomal iron) has been developed, which associates a high bioavailability with a low incidence of side effects and which has been shown to be non-inferior than a typical dosing strategy with Intravenous iron gluconate in patients with CKD not on dialysis.14,20 However, a limitation of that study was the high selection of patients in the 2 groups compared, which limits the extrapolation of the results to the entire population of patients with CKD.14
The objective of the present study was to evaluate the effect of sucrosomial iron in the management of iron deficiency anemia in patients with moderate CKD (stages 3a and 3b), GI limitation to conventional oral iron therapy and with broader selection criteria of patients than facilitate the extrapolation of results to the population of patients with moderate CKD.
Material and methodsPatientsPatients recruited were on treatment and follow-up in the outpatient clinics at the Hospital Clínic de Barcelona. The predetermined inclusion criteria were: (a) the presence of moderate stable CKD (stages 3a and 3b, defined according to the KDIGO 2012 guidelines) and (b) the presence of GI limitation or intolerance prior to oral iron therapy.
The Ethical Committee for Medical Research of the Hospital Clínic de Barcelona approved this observational study with the reference HCB-2016–0520.
Study designThe primary objective of the present study was to evaluate the efficacy of the treatment with sucrosomial ferric pyrophosphate (FeSu) in patients with stage 3 CKD, evaluated as an increase in baseline hemoglobin (Hb) levels at 6 and 12 months of treatment. In addition, other parameters related to iron metabolism were analyzed, as well as tolerance and therapeutic adherence to the new preparation. The patients renal function was also evaluated.
To assess the therapeutic impact on anemia and iron metabolism the following parameters were measured: Hb, mean corpuscular volume, mean corpuscular hemoglobin and the percentage of hypochromic red cells. In addition, certain parameters inherent to iron metabolism were also measured, such as serum ferritin, the transferrin and transferrin saturation index (TSI). Another aspect evaluated during follow-up was therapeutic adherence and the appearance of adverse effects, as well as therapeutic satisfaction (using a Likert scale). Poor therapeutic adherence was defined as the voluntary omission of at least one oral iron tablet for 3 or more days weekly.
Monitoring and evolution of renal function was performed by measuring the estimated glomerular filtration rate (eGFR) using the CKD-EPI formula (Chronic Kidney Disease-Epidemiology Collaboration), urine albumin/creatinine ratio, serum albumin and intact parathormone (PTH). The inflammatory state of the patients was monitored by measuring C-reactive protein and serum albumin.
Following recruitment, patients included received a FeSu dose of 30mg/day orally over a 12-month period. During this period, the clinical and analytical parameters listed above, as well as therapeutic adherence, were monitored and measured at 3 points: at the start of the study (time zero or T0), at 6 months (T1) and at 12 months of treatment (T2). Finally, a comparative study of the values of these variables was carried out at 6 and 12 months with respect to their baseline values prior to the start of treatment.
Statistic analysisQualitative variables were described using frequencies and percentages. Quantitative variables were expressed as mean and standard deviation. The generalized estimation equation method was used to study the evolution of laboratory tests. Furthermore, an interchangeable correlation structure was assumed for intrapatient observations. The variance-covariance matrix of the regression coefficients was estimated using a robust sandwich variance estimator. The level of significance was set at 0.05. The analysis was performed using the R v.3.0.5 program (R Foundation for Statistical Computing, Vienna, Austria).
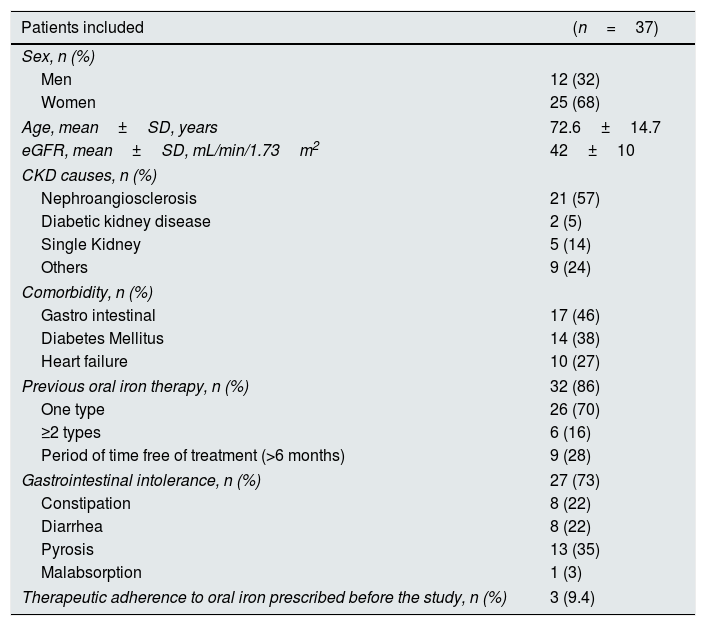
ResultsBaseline dataTable 1 summarizes the baseline characteristics of the patients included in the study.
Baseline characteristics of the patients included in the study.
| Patients included | (n=37) |
|---|---|
| Sex, n (%) | |
| Men | 12 (32) |
| Women | 25 (68) |
| Age, mean±SD, years | 72.6±14.7 |
| eGFR, mean±SD, mL/min/1.73m2 | 42±10 |
| CKD causes, n (%) | |
| Nephroangiosclerosis | 21 (57) |
| Diabetic kidney disease | 2 (5) |
| Single Kidney | 5 (14) |
| Others | 9 (24) |
| Comorbidity, n (%) | |
| Gastro intestinal | 17 (46) |
| Diabetes Mellitus | 14 (38) |
| Heart failure | 10 (27) |
| Previous oral iron therapy, n (%) | 32 (86) |
| One type | 26 (70) |
| ≥2 types | 6 (16) |
| Period of time free of treatment (>6 months) | 9 (28) |
| Gastrointestinal intolerance, n (%) | 27 (73) |
| Constipation | 8 (22) |
| Diarrhea | 8 (22) |
| Pyrosis | 13 (35) |
| Malabsorption | 1 (3) |
| Therapeutic adherence to oral iron prescribed before the study, n (%) | 3 (9.4) |
CKD: chronic kidney disease; eGFR: estimated glomerular filtration.
The study include a total of 37 patients (25 women and 12 men) with a mean age of 72.6±14.7 years and with a previous diagnosis of CKD stage 3 (mean eGFR 42±10mL/min/1.73m2). The most frequent cause of CKD was nephroangiosclerosis (57%). A high rate of comorbidities was documented among the subjects included in the study: 17 patients (46%) reported GI comorbidity, 14 patients (38%) had diabetes mellitus, and 10 (27%) were diagnosed with heart failure.
Of the 37 patients included, 32 patients (86%) had received previous oral iron treatment with iron salts and/or ferrimanitol ovalbumin: 26 patients (70%) had been treated with a single type of oral iron and 6 (16%)) with 2 or more types. Of the 32 patients previously treated with oral iron, 9 (28%) had a treatment-free period (before the initiation of the study) of more than 6 months.
Of the total number of patients included in the study, 27 (73%) manifested some type of gastrointestinal limitation or intolerance to standard oral iron therapy: constipation (8 patients, 22%), diarrhea (8, 22%), heartburn (13 patients, 35%) or malabsorption (one patient, 3%). Before starting the study the therapeutic adherence of patients with prior prescription of oral iron was 9.4%.
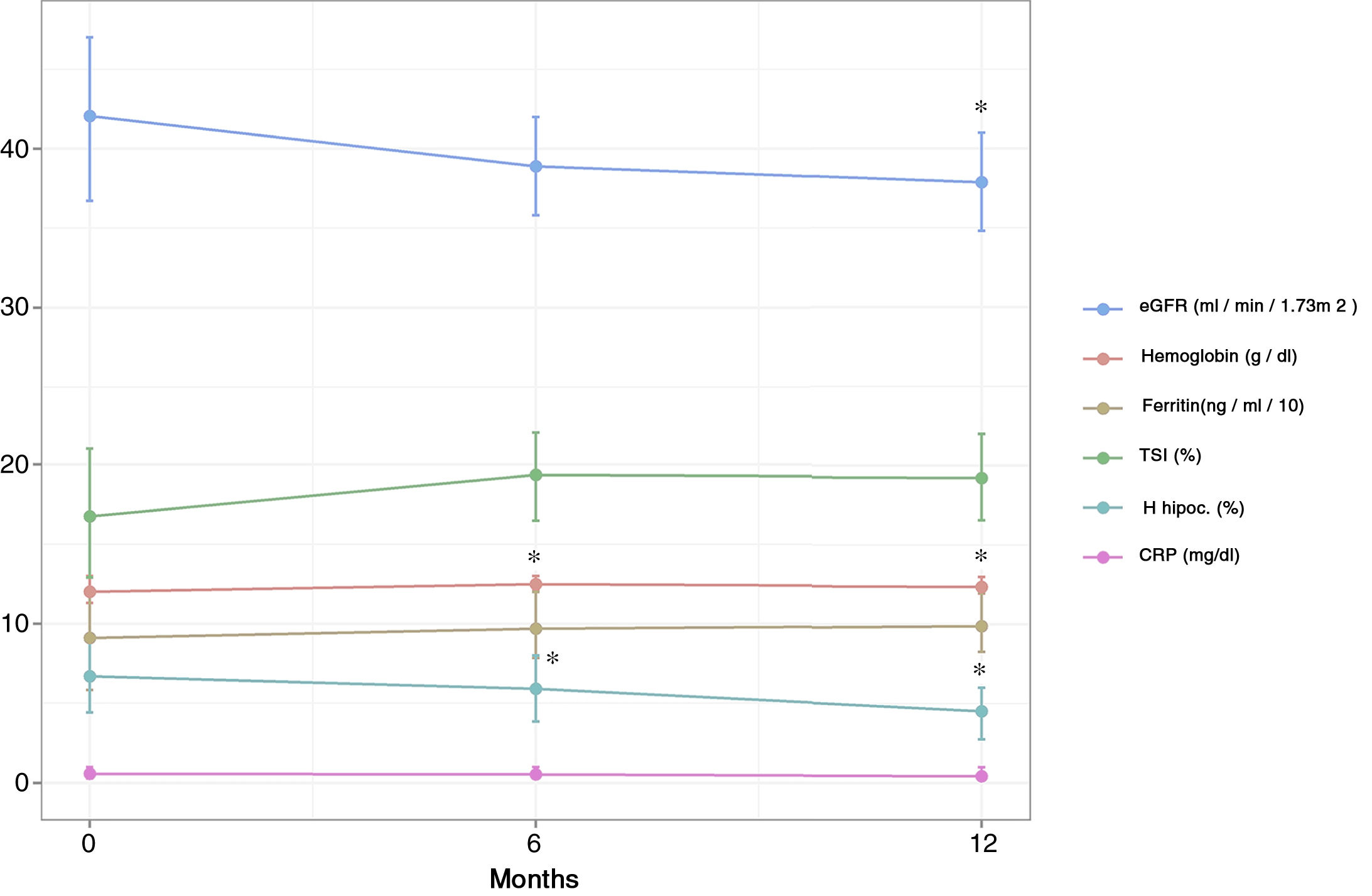
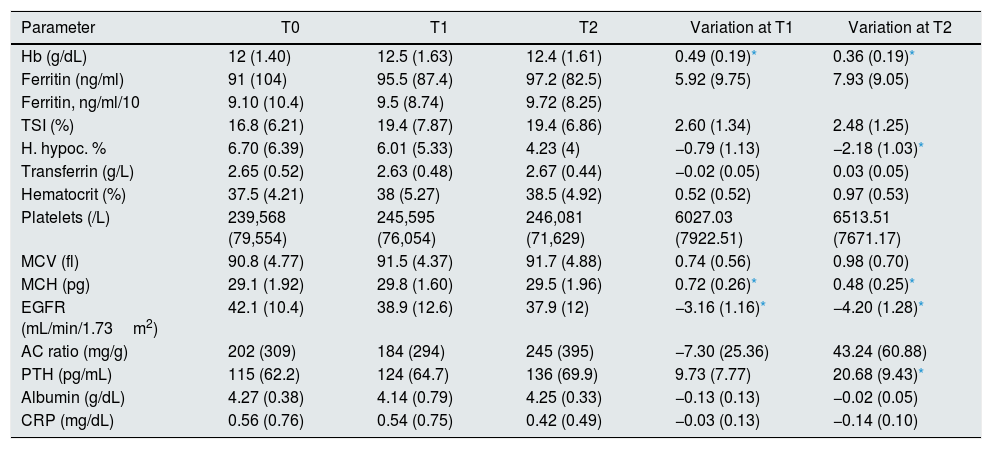
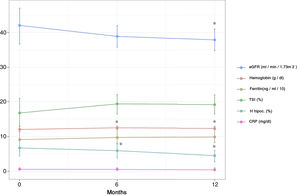
Variation of the parameters analyzed during the follow-upAfter 6 months of treatment, a significant increase in Hb values was observed with respect to the baseline value, an increase that was maintained after 12 months of treatment (0.49±0.19 and 0.36±0.19g/dL at T1 and T2, respectively) (Table 2 and Fig. 1). This increase was associated with a statistically significant increase in mean corpuscular hemoglobin at 6 months that was maintained until 12 months (increase of 0.72±0.26 and 0.48±0.25pg at T1 and T2, respectively). The percentage of hypochromic red cells did not decrease significantly at 6 months, however a significant decrease was observed at 12 months of treatment (reduction of 0.79±1.13% and 2.18±1.03% at T1 and T2, respectively). The rest of the hematological and iron metabolism parameters analyzed (ferritin, IST, transferrin, hematocrit, platelets, and mean corpuscular volume) did not show significant variations with respect to the baseline value in the 2 moments analyzed, although in the case of ferritin and IST a non-significant increase was recorded at 6 and 12 months (Table 2 and Fig. 1).
Values of the parameters analyzed at the beginning of the study and during follow-up.
| Parameter | T0 | T1 | T2 | Variation at T1 | Variation at T2 |
|---|---|---|---|---|---|
| Hb (g/dL) | 12 (1.40) | 12.5 (1.63) | 12.4 (1.61) | 0.49 (0.19)* | 0.36 (0.19)* |
| Ferritin (ng/ml) | 91 (104) | 95.5 (87.4) | 97.2 (82.5) | 5.92 (9.75) | 7.93 (9.05) |
| Ferritin, ng/ml/10 | 9.10 (10.4) | 9.5 (8.74) | 9.72 (8.25) | ||
| TSI (%) | 16.8 (6.21) | 19.4 (7.87) | 19.4 (6.86) | 2.60 (1.34) | 2.48 (1.25) |
| H. hypoc. % | 6.70 (6.39) | 6.01 (5.33) | 4.23 (4) | −0.79 (1.13) | −2.18 (1.03)* |
| Transferrin (g/L) | 2.65 (0.52) | 2.63 (0.48) | 2.67 (0.44) | −0.02 (0.05) | 0.03 (0.05) |
| Hematocrit (%) | 37.5 (4.21) | 38 (5.27) | 38.5 (4.92) | 0.52 (0.52) | 0.97 (0.53) |
| Platelets (/L) | 239,568 (79,554) | 245,595 (76,054) | 246,081 (71,629) | 6027.03 (7922.51) | 6513.51 (7671.17) |
| MCV (fl) | 90.8 (4.77) | 91.5 (4.37) | 91.7 (4.88) | 0.74 (0.56) | 0.98 (0.70) |
| MCH (pg) | 29.1 (1.92) | 29.8 (1.60) | 29.5 (1.96) | 0.72 (0.26)* | 0.48 (0.25)* |
| EGFR (mL/min/1.73m2) | 42.1 (10.4) | 38.9 (12.6) | 37.9 (12) | −3.16 (1.16)* | −4.20 (1.28)* |
| AC ratio (mg/g) | 202 (309) | 184 (294) | 245 (395) | −7.30 (25.36) | 43.24 (60.88) |
| PTH (pg/mL) | 115 (62.2) | 124 (64.7) | 136 (69.9) | 9.73 (7.77) | 20.68 (9.43)* |
| Albumin (g/dL) | 4.27 (0.38) | 4.14 (0.79) | 4.25 (0.33) | −0.13 (0.13) | −0.02 (0.05) |
| CRP (mg/dL) | 0.56 (0.76) | 0.54 (0.75) | 0.42 (0.49) | −0.03 (0.13) | −0.14 (0.10) |
AC ratio urine albumin/creatinine ratio; EGFR: estimated glomerular filtration rate; H. hypoc: hypochromic red cells; Hb: hemoglobin; MCH: mean corpuscular hemoglobin; TSI: transferrin saturation index; CRP: C reactive protein; PTH: parathormone; T0: start of the study (baseline); T1: 6 months after starting treatment with sucrosomed iron; T2: 12 months after starting treatment with sucrosomed iron; MCV: mean corpuscular volume.
The parameters are expressed as mean (SD).
Variation of the main hematological parameters and in the GFR throughout the follow-up (6 and 12 months) after the start of treatment. eGFR: estimated glomerular filtration rate; H hipoc. (%): RBCs hipocrom; TSI: transferrin saturation index; CRP: C reactive protein. *p<0.05 with respect to the basal value (T0).
There was a mild, although significant, reduction in eGFR during the follow-up, (3.16±1.16 and 4.20±1.28mL/min/1.73m2 at T1 and T2, respectively). Serum PTH concentration did not show a significant variation at 6 months, although a significant increase was observed at 12 months of treatment. Other parameters such as albumin, urine albumin/creatinine ratio and C-reactive protein did not show significant variations at the end of the follow-up period (Table 2 and Fig. 1).
Adverse reactions and therapeutic adherenceNone of the patients included in treatment with FeSu presented adverse reactions or adverse gastrointestinal effects that forced to discontinue the treatment at 6 or 12 months.
The therapeutic compliance rate was 100% in the time periods analyzed (6 and 12 months after the start of treatment). The results of the Likert scale were in great agreement, 1, or in agreement, 2, with the therapeutic satisfaction in the total group of patients.
DiscussionSupplementing with iron constitutes a fundamental component of the anemia treatment in patients with CKD. In patients with CKD on dialysis, intravenous iron therapy has clearly demonstrated its efficiency with respect to the oral route.14 In patients with CKD not on dialysis, the superiority of the intravenous route over the oral route is a matter of debate, although some studies have shown that intravenous iron therapy is superior to oral iron in achieving greater repletion of iron deposits, and also, although to a lesser extent, a greater increase in Hb15–18 however in this condition the preservation of the vascular tree is a gold standard.
Theoretically, the lowest effectiveness of oral vs. IV Iron is justified by its low bioavailability and lower adherence related mainly to gastrointestinal side effects associated with their administration.14,19 In an attempt to overcome these difficulties, the so-called liposomal or sucrosomed iron has been commercialized, a new generation oral iron compound that, due to its pharmacological design incorporating a phospholipid envelope, exhibits a greater bioavailability than conventional oral preparations. In addition to a significantly lower rate of gastrointestinal adverse effects.14,20 Recently, Pisani et al.14 carried out a randomized clinical trial in which liposomal iron proved to be no less effective than intravenous iron in terms of increasing Hb levels after 3 months of treatment, although IV iron proved to be superior in achieving greater degree of repletion of iron stores (ferritin, TSI) and a more rapid and sustained increase in Hb levels at the end of treatment.
One of the main limitations of the study by Pisani et al.14 was the high degree of selection of the patients included in each of the 2 study groups, a fact that limits the validity of the study and, therefore, the extrapolation of the results to the population of CKD patients not on dialysis. The present study was designed with a primary objective, to evaluate the efficacy of liposomal iron to increase Hb levels, and determine the degree of tolerance and therapeutic adherence of patients with stage 3 CKD, with less strict inclusion criteria.
In our cohort of patients treatment with liposomal or sucrosomed iron was associated with a significant increase in Hb levels, already detectable at 6 months (increase of 0.49±0.19g/dL compared to baseline) and it was maintained at 12 months of treatment (increase of 0.36±0.19g/dL). These results are in agreement with those obtained by Pisani et al.,14 although in such study the follow-up was only 3 months. However, the parameters that reflect repletion of the iron deposits did not change significantly with respect to their baseline values, just as observed in the present study. This phenomenon has been attributed to ascorbic acid associated with liposomal iron, which favors the release of iron associated with ferritin and mobilizes it from the reticuloendothelial system to its transport by transferring.21 The analysis of the inflammatory state of the cohort of treated patients, measured by the levels of C-reactive protein and albumin, did not show significant changes throughout the follow-up that could potentially affect the absorption of oral iron and its bioavailability mediated by the hepcidin and consequently on Hb levels.
None of the study patient required starting treatment with erythropoiesis-stimulating agents, despite the fact that renal function decreased significantly in the period studied (decrease in eGFR with respect to the baseline value of 3.16±1.16 and 4.20±1, 28mL/min/1.72m2 at 6 and 12 months, respectively). Associated with this reduction in eGFR, a significant increase in PTH figures was observed 12 months after treatment. There are studies that have describe the potential negative effect of intravenous iron preparations on renal function, especially in terms of tubular damage and proteinuria.22–24 These effects have not been described in studies using oral iron for the treatment for anemia in patients with CKD.14 In the present study, the comorbidity of the patients included in the study could favor the progression of CKD, which is relatively slow (less than 5mL/min/year). In this sense, the increase in PTH levels and the worsening of the eGFR may have acted as confounding factors, minimizing the effect of liposomal iron on Hb levels.25
One of the key findings of the present study has been the excellent tolerance to oral treatment with liposomal or sucrosomed iron, similar to what has been reported by Pisani et al.14 One main limitations of the oral treatment of iron is the high frequency of adverse reactions leading to a high rate of abandonment of the treatment.19 This poor therapeutic adherence ultimately leads to low efficacy of oral treatment. In our cohort of patients who had previously received supplementation with classical oral iron compounds the therapeutic adherence was extremely low (9.4%). However, with the initiation of treatment with liposomal iron, therapeutic adherence was 100% at 6 and 12 months, with no adverse reactions that could compromise therapeutic compliance. The absence of adverse reactions associated with iron liposomal implies a distinct advantage over other oral compounds, but also regarding intravenous preparations, which are not free of complications associated with infusion.14,19
Although the results obtained in the present study about the effectiveness of liposomal iron are promising in this patient population, its limitations must be taken into account. The main limitation is the small number of patients included, a fact that forces us to interpret the results with caution. Despite these limitations, this study offers preliminary evidence that should be used for future randomized, controlled studies with a larger number of patients, to provide stronger evidence of the effectiveness and adverse effects of liposomal iron in patients with moderate CKD.
In conclusion, the present study demonstrates the efficacy of liposomal or sucrosomed iron to increase Hb levels in a cohort of patients with CKD stage 3 that can be extrapolated to the general population of patients with moderate CKD. The low rate of adverse reactions and the excellent tolerability to liposomal iron place this compound as a first-line in the treatment of anemia in patients with CKD, especially in those patients with intolerance to classic oral treatment. However, as previously mentioned, more studies are needed to evaluate, in a randomized and controlled manner, the efficacy of liposomal iron as compared to the classic compounds, and with respect to intravenous therapy.
AuthorshipPA and RA have designed and actively contributed to the project that was approved by the Medical Research Ethics Committee of Hospital Clínic as an observational study with reference HCB-2016-0520.
All authors have contributed to the writing of the manuscript, approving the final version and sending it for publication in Nefrologia.
Conflicts of interestThe authors declare that they have no conflict of interest.
Preliminary results at 6 months of treatment were presented at the 5th International Multidisciplinary Course on Iron Anemia. Firenze 31.3-1.4.2017.
Please cite this article as: Montagud-Marrahi E, Arrizabalaga P, Abellana R, Poch E. Hierro liposomal en la enfermedad renal crónica moderada. Nefrologia. 2020;40:446–452.