Desensitization is a procedure undergone by the recipient of a kidney transplant from a donor who is cross-match positive. The aim of this study was to present the outcomes from our hospital of kidney transplant recipients from HLA-incompatible live donors after desensitization.
We studied 32 patients aged 46±14 years with a mean fluorescence intensity (MFI) versus class I HLA of 7979±4089 and 6825±4182 MFI versus class II and relative intensity scale (RIS) of 8.9±7.6. The complement-dependent cytotoxicity (CDC) cross-matching test was positive in 18 patients, flow cytometry was positive in 7 patients and donor-specific antibodies (DEA) were detected in 7. The protocol used was rituximab, plasmapheresis/immunoadsorption, immunoglobulins, tacrolimus, mycophenolic acid derivatives and prednisone.
After 8±3 sessions of plasmapheresis/immunoadsorption, 23 patients were transplanted (71.9%) and desensitization was ineffective in 9. There were baseline differences in MFI class I (p<0.001), RIS (p=0.008), and CDC cross-matching, DSA and flow cytometry (p=0.05). MFI class I and RIS were predictors of inefficiency in ROC curves. After follow-up of 43±30 months, 13 patients (56%) presented postoperative bleeding, 3 (13%) delayed graft function, 4 (17.4%) acute rejection, 6 (26%) CMV viremia and 1 (4%) BK viremia. Five-year patient survival was 90%, with 86% allograft survival. Five-year creatinine was 1.5±0.4 and proteinuria was 0.5±0.7.
ConclusionsKidney transplantation from HLA-incompatible live donors after desensitization was possible in 71.9% of patients. MFI class I and RIS predict the inefficiency of desensitization. Five-year allograft survival (86%) was acceptable with a low incidence of acute rejection (17.4%), although with a greater trend toward postoperative bleeding.
La desensibilización es un método empleado en trasplante renal para tratar de trasplantar a pacientes que presentan una prueba cruzada positiva frente a su donante. El objetivo del estudio es mostrar los resultados de nuestro hospital en pacientes trasplantados renales con donantes vivos HLA incompatibles, tras un protocolo de desensibilización.
Estudiamos a 32 pacientes de 46±14 años que presentaban una intensidad media de fluorescencia (MFI) frente a HLA de clase I de 7.979±4.089 y de 6.825±4.182 para MFI frente a clase II y relative intensity scale (RIS) de 8,9±7,6. La prueba cruzada fue positiva por citotoxicidad dependiente del complemento (CDC) en 18 pacientes, citometría de flujo (CF) en 7 y anticuerpos donante específicos (ADE) en 7. El protocolo empleado fue: rituximab, plasmaféresis/inmunoadsorción, inmunoglobulinas, tacrolimus, derivados ácido micofenólico y prednisona.
Tras 8±3 sesiones de plasmaféresis/inmunoadsorción se trasplantó a 23 pacientes (71,9%) y resultó ineficaz en 9. Existían diferencias basales en MFI clase I (p<0,001), RIS (p=0,008) y cross-match por CDC, ADE o CF (p=0,05). El MFI de clase I y el RIS resultaron predictores de eficacia en curvas COR. Tras un seguimiento de 43±30 meses, 13 pacientes (56%) presentaron sangrado postoperatorio, 3 (13%) función retrasada injerto, 4 (17,4%) rechazo agudo, 6 (26%) viremia CMV y uno (4%) viremia BK. Al 5.° año, la supervivencia del paciente fue del 90% y la supervivencia renal del 86%. En ese mismo año, la creatinina fue de 1,5±0,4 y la proteinuria de 0,5±0,7.
ConclusionesEl trasplante renal de donante vivo HLA incompatible tras la desensibilización fue posible en el 71,9% de los pacientes. MFI de clase I y RIS predicen ineficacia de la desensibilización. La supervivencia renal (86% al 5.° año) es aceptable con baja incidencia de rechazo agudo (17,4%), aunque con una mayor tendencia al sangrado postoperatorio.
Patients that have developed hypersensitivity (HY) have increasing difficulty to access to a kidney transplant because of the high incidence of pre-transplant positive cross-match. In USA, 35% of the patients on the waiting list for renal transplantation are sensibilized and 15% are highly sensibilized.1 Recently, it has been reported that 6% of patients on the waiting list have a 100% of calculated reactivity against the panel.2 In Spain, in 2014, 11.5% of patients on the transplant waiting list are HY.
The remedy for these patients is to use desensitization (DS) techniques, inclusion in either cross-transplant lists (living donor) or in exclusive list of cadaveric donor for sensibilized patients.3–6 Survival is better in DS patients than in those who remain on the waiting list and are transplanted with a compatible donor and it is even better than those in the list that remain on dialysis.7 Two review manuscripts compare the results of different DS techniques: Immunoglobulins (IgV), high dose IgV, plasmapheresis (PF) or immunoadsorption (IA) with or without IgV, rituximab (RTX), bortezomib, but there is great heterogeneity in: patient selection (live donor versus cadaver), the method used for cross-match selection (complement dependent cytotoxicity [CDC], flow cytometry [FC], specific donor antibodies [SDA] by Luminex), follow up and type of monitorization to assess the response to desensitization. In these groups of transplant patients the rate of rejection varies from 0% to 100% – and depends on the treatment used and the selection criteria. Patient survival ranges from 80 to 100% and graft survival between 69 and 100%. In 2014, Riella et al., using a DS protocol based on PF, IgV and RTX, reported a 61% of antibody-mediated rejection rate, after 5 years the patient survival was 86% and the renal survival was 84%.9
In 2012, within our area of health care, there were only 24 transplanted patients who presented reactivity against the panel of 70%. This group of patients had been in the transplant waiting list for the longest period of time.10 Only 3 patients were transplanted under the network renal transplant program; the National Plan of Renal Exchange for Hyperimmunized Patients (PATHI) was not started in Spain until 2015. Therefore, taking into consideration the results of the other series above described, we started a DS program in 2007, the objective was to open up the possibility of transplantation to this group of patients. The aim of the present study is to show our results obtained from “A Coruña University Hospital” in patients, with HLA incompatible living donors, who underwent desensitization (DS).
MethodsThe study began in January 2007 and it was completed in 2014. During this period, 100 patients received a kidney transplant from a living donor. We evaluated 32 patients on pre-transplantation DS protocol; 21 of them were on hemodialysis, 5 in peritoneal dialysis, and 6 were in predialysis. All patients had a positive cross-reactivity against their donor as assessed by CDC, FC or Luminex. Prior to the onset of DS, patients and donors were informed about the procedure and gave their official consent form authorized in our center. In addition, the donors signed the consent in court, according to the protocol of live donation.
DesensitizationThe DS protocol consisted in administration of RTX 375mg/m2 (Roche Farma AG, Grenzach-Wyhlen, Germany) one month prior to transplantation. Tacrolimus, MMF or MFS and prednisone were initiated one week before starting PF/IA. PF or non-specific IA was performed initially on a daily basis, except on Sundays. IA was performed in 19 patients using Therasorb columns, PF in 12 patients and in one patient both procedure were used. After each PF/AI session the patients received specific anti-CMV IgV 100mg/kg (Cytotect Biotest Pharma GmbH, Germany). Hemodialysis was performed every 48h after the apheresis session or 4 daily exchanges of peritoneal dialysis. Once the apheresis sessions were started, we either monitored the antibody count or performed cross-reaction tests. When CDC or FC cross-reaction test was negative and SDA levels reached a mean fluorescence intensity (MFI) of less than 1000, the patient was accepted for transplantation. Once transplanted, PF or IA was performed on days +3, +5 and +8 after transplantation.
ImmunosuppressionInduction with basiliximab was performed in 13 patients (56.5%). Tacrolimus levels were set at 10–12ng/ml during the first week, and were reduced progressively to reach 7–8ng/ml from the 6th month. The initial dose of MMF (Cellcept) was 2g/day, followed by reduction of 1g/day per month. The dose of MFS (Myfortic) was 1440mg per day, which was reduced to 720mg/day per month. Prednisone was started at 20mg/day, and it was progressively reduced to 5mg by month 6. Prophylaxis was performed using co-trimoxazole for 6 months, itraconazole for 4–6 months, and valganciclovir (Valcyte; Roche Farma, Madrid, Spain) in CMV donor positive patients, CMV negative receptor (Fig. 1).
Follow upAfter discharge, patients were followed in the out patient clinic with monitorization of renal function, tacrolimus levels, CMV infection first with pp55 antigenemia and presently using PCR of CMV and BK virus.
If deterioration of renal function was evidenced, a biopsy was obtained and SDA were determined. In our transplant unit protocol biopsies or systematic follow-up of SDA are not performed.
The diagnosis of rejection in the biopsy was made based on Banff classification, adapted to the date in which this classification was published.11,12
Antibody-mediated rejection was treated with steroids (3 boluses of 500mg for 3 consecutive days), PF or IA, IgV, RTX and eculizumab (in refractory cases). Treatment of T-cell mediated rejection included steroid boluses (3 boluses of 500mg for 3 consecutive days).
Variables evaluated: Efficacy of DS, delayed graft function, acute rejection, graft and patient survival, renal function, number of procedures of PF/IA, MFI against class I and II pre and postapheresis, Number of antibodies against class I and II, relative intensity scale (RIS) as reported by Jordan et al.,10 CMV and BK infections, diabetes posttransplant, neoplasias and urological complications.
E-statistics analysis: Quantitative variables were compared by Student t test and ANOVA. Mann–Whitney was used to compare variables without normal distribution.
Chi square and Fisher's test was used for qualitative variables. Survival was calculated according to Kaplan–Meier and log rank test. For the multivariate study we used the Cox regression and for the sensitivity and specificity analysis, the ROC curves. The statistical analysis was performed using the program SPSS (version 15.0.1, Chicago, IL, USA).
ResultsThe study included 32 patients (15 males), with a mean age of 46±14 years. The relationship between donor and recipient was: parent,9 spouse,14 sibling,5 child1 and others.3
At baseline, patients presented a number of antibodies against class I: 0.9±0.7 (0–3) with MFI 7979±40 and class II: 0.6±0.5 (0–2) with MFI 6825±4182; RIS 8.9±7.7 (2–30). CDC cross-match was positive in 18 patients; by FC, in 7. Another 7 patients had only SDA using solid phase techniques (Luminex, One Lambda, Thermo Fisher Scientific, Canoga Park, CA, USA). DS was considered effective when CDC and FC cross-tests were negative and the MFI less than 1000 (Table 1).
Desensitization efficacy.
| Efficacy: n=23 | Without efficacy: n=9 | p | |
|---|---|---|---|
| Age of donor (years) | 50.3±9 | 49.6±6 | NS |
| Age of recipient (years) | 48.6±14 | 38.6±12 | 0.07 |
| Dialysis vintage (months) | 84.9±77 | 39.3±46 | 0.07 |
| No. of antibodies class I and II | 1.47±0.79 | 1.66±0.70 | NS |
| MFI class I | 5840±3002 | 12,258±2044 | 0.001 |
| MFI class II | 6048±4145 | 9169±3725 | NS |
| RISa | 6.2±6.6 | 15±7 | 0.005 |
| Pregnancy | 0.86±1 | 0.33±0.07 | NS |
| No. of HLA incompatibilities | 3.7±11 | 3.3±1.6 | NS |
| Number of PFS/IA sessions | 8.1±2.7 | 8.7±3.3 | NS |
| Cross-match: CDC/FC/SDA (n°)b | 10/7/6 | 8/0/1 | 0.02 |
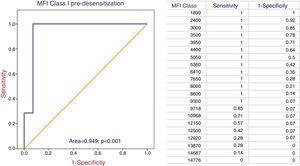
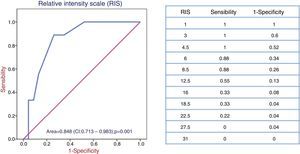
After 8±3 sessions of PF/IA4-15, it was possible to perform transplantation in 23 patients (71.9%), but in 9 patients the transplant failed. Desensitization was not successful in 9 patients; 8 of these patients presented a previous cross-match by CDC and one by SDA (p=0.02). Class I MFI and RIS were predictors of efficacy by ROC curves (area of 0.949 and 0.848, respectively). A cutoff point of MFI and RIS was set at 9300 and 8.5 points respectively, with provides 100% sensitivity and 93% specificity for the first and 88% sensitivity and 74% specificity for the second. DS was not effective above these values (Figs. 2 and 3).
In the postoperative period, 13 patients had hematoma-hemorrhage, requiring reintervention in 6 (26%). Blood transfusion was necessary in 16 (69%). In 3 patients (13%) there was a delay the initial function of the graft.
After 43±30 months (range 0.1–104) of follow-up, 4 patients (17.4%) had acute rejection; in one patient it was antibody-mediated, in two T-mediated and in the last of four it was mixed (antibodies and T cells mediated rejection). Patients with rejection had a higher baseline MFI, higher values of RIS, and a positive CDC cross-match (Fig. 4), but in the Cox analysis, RIS was the only parameter that reached statistical significance.
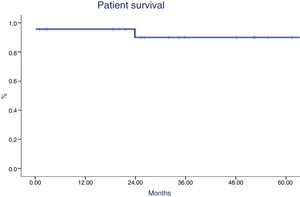
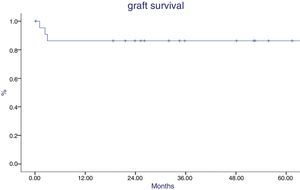
Patient survival at 1 and 5 years were 96% and 90% respectively (Fig. 5). One patient died during sepsis of urinary origin (during the first week postransplantation) and one died after subarachnoid hemorrhage (during the second year). Graft survival was 86% at year 1 and 5 after transplantation (Fig. 6), being worse in patients with acute rejection mediated by antibodies (p=0.02). The causes of graft loss were antibody-mediated rejection in 2 patients (one of them also had T cell mediated rejection), chronic nephropathy in one patient, and arterial thrombosis in another.
The mean serum creatinine (mg/dl) was 1.2±0.3, after one year, 1.4±0.4 the 3rd year and 1.5±0.04at the end of the 5 years of follow-up. Mean values of proteinuria (mg/day) at years 1, 3 and 5 post transplantation were 0.3±0.3, 0.2±0.2 and 0.5±0.7 respectively
There were six CMV infections (26%) and one BK viremia (4%). The frequency of post-transplant diabetes was 26%,6 lymphocele 13%3 and renal artery stenosis 4%.1
Two post-transplant neoplasia were diagnosed, one chronic lymphatic leukemia and one cutaneous squamous cell carcinoma.
DiscussionRenal transplantation is the best therapeutic option for patients with end stage renal disease.14 In hypersensitized patients the rate of transplantation is low (<6.5% per year in USA, up to the last year), they remain in the waiting list for long periods of time and the mortality is high. The options of a patient with an incompatible living donor are DS, joining cross-renal donation programs which is valid option if a living donor is available, or being included in special exchange and prioritization programs.3–6
Our DS protocol is similar to others being used during the last years by different groups. However there is a lot of heterogeneity between the series, especially when defining selection criteria for desensitization and transplantation.1,8 In our serie, cross-match was positive for CDC in 18 of the 32 patients and it was not possible to desensitize 44% of them. The results were more favorable in those who presented a positive cross-reaction by FC or only with SDA by Luminex, in fact we managed to transplant 100 and 86% of those respectively. Orandi et al. showed that DS improves the prognosis of patients as compared with those who remain on the waiting list and finally are transplanted or those remaining on dialysis. These differences were maintained for any level of baseline DS (CDC+, CF+ or only SDA+ by Luminex), although the prognosis was worse in those with cross-reaction test was positive by CDC,7 which could mean that these patients positive by CDC have a higher number of antibodies or higher affinity and this allows them to be detected by techniques that are less sensitive with respect to CF or Luminex.3
Another factor that could influence the results is the amount of antibodies, interpreted as the level of MFI, which is not well known in most of the series. We used RIS to homogenize the number of antibodies in each patient and their MFI, as described by Jordan et al.13 Our results demonstrate that DS was not effective in patients with MFI greater than 9300 in class I, or in those with an RIS greater than 8.5.
In our study, at year 5 the survival of patient (90%) and graft (86%) was similar to that reported by others and illustrate the possibility of performing this type of treatment in HY patients who would otherwise remain for a long time in the waiting list. Marfo et al.1 reports a 95% patient survival and 85% graft survival; Orandi at year 3, 91.7% and at year 8 a 76.5%6; Yang et al. in Taiwan,15 at year 5, they report 100% of the patient and 92.3% of the graft survival; and De Sousa et al., 95.9% of patients and 85% of grafts survived after 5 years.16 Morath et al.,17 using PF or AI, reported a 95.8% patient survival and 92.4% graft survival, after one year. Bentall et al.18 reported a 5-year patient survival rate of 83.5%, lower than in the control group (92.5%) and a graft survival of 70.7%, lower than in the control group (88%), and Thielke et al.19 showed 95% patient survival and 93% of the graft survival after one year.
The incidence of acute rejection was 17%, in 2 cases antibody-mediated (AMR), one of them also had T-cell mediated rejection with no response to treatment, and 2 other patients had rejections mediated only by T cells, Banff IA, with good response to steroids. In our serie, the probability of rejection was greater in patients with CDC-positive cross-match and in those with higher RIS. Our low frequency of rejection contrasts with others that have reported from 17 to 80% rejections, using different protocols and different degrees of DS (CDC, CF, SDA).1,8 We do not perform biopsies by protocol and only monitor SDA if there is a suspicion of antibody-mediated rejection, so we may not have detected subclinical rejections or chronic rejections mediated by antibodies, which could explain this difference. Riella et al that uses a DS protocol similar to ours,9 has reported a 61% rejection; 100% of patients had CDC-positive cross-match pre-transplantation and the monitorization of titers differs from ours in that we used MFI and they used antibody titers; in addition, only 21 of 39 received RTX. After DS with bortezomib, Aubert et al.20 found no acute rejection after 18 months and Woodle et al.21 reported 31%. Recently, Moreno Gonzales et al.,2 using 32 doses of bortezomib only found moderate reduction of antibody without negativization of the cross-match, and it was not well tolerated.
There is little information on the incidence of post-transplant SDA in the series of desensitized patients. Morath et al.17 monitored antibodies during one year and these remained positive at low titers. De Sousa et al.16 monitored the antibodies in 18 patients, in one of them were positive and developed AMR. Kauke et al.22 demonstrated that in 6 out of 8 desensitized patients the antibodies persisted and 3 of them developed AMR. Stegall et al.,23 in patients treated with eculizumab, found that 50% of the patients developed SDA with MFI >10,000, although the incidence of acute rejection was 7.7% as compared with 41.1% in the group without eculizumab. In our series, 8 patients had SDA at follow-up: 3 of them were positive, 2 developed AMR and another patient had transplant glomerulopathy.
In our study, despite the high load of immunosuppression, the rate of infectious complications was acceptable, with an incidence of CMV of 26% and BK of 4%. Vo et al.24 reported a 16.5% CMV infection and 0% BK viremia. Kauke et al.,22 reported 2/8 (25%) of BK viremia. Yang et al.15 reported 3 cases of BK nephropathy (13.6%) and 2 CMV pneumonitis (9%). De Sousa et al.16 reported 3 CMV reactivations (13%) and one case of BK (4.3%). Morath et al.17 describe 10% CMV and 0% BK. Thielke et al.19 7% CMV and 4.9% BK. Stegall et al.23 used eculizumab without reporting no CMV or BK virus infections.
The incidence of neoplasias is not usually reported. Two patients in our series had de novo neoplasia (a chronic lymphatic leukemia and a skin squamous cell carcinoma). The Taiwan group reported 3 cases of urothelial cancer. Stegall et al., in patients desensitized with eculizumab, reported one case of Burkitt's lymphoma.
A relatively frequent complication following transplantation is the occurrence of hematoma/hemorrhage. It could be related to PF or IA in incompatible ABO transplants due to the loss of coagulation factors, including factor XIII, the decrease in the number of platelets or the use of anticoagulants to perfuse the graft.25–27 In our patients we did not identify a factor that could favor bleeding (although we did not measure factor XIII), perhaps due to the limited number of patients. There might be a number of factors that acting together may facilitate hemorrhage.
The limitations of our study are the sample size, the absence of protocol biopsy and the low number of SDA monitored. Despite this, the clinical results are acceptable in a group of patients who would have to be maintained on the waiting list for a long time. We detected markers that identify patients in whom DS is ineffective, these are MFI class I or RIS, which would suggest to avoid the initiation a costly treatment with potential adverse effects. We conclude that the HLA DS with the protocol shown in this study is an option to consider in those patients sensitized against a living donor.
Conflict of interestsThe authors have no conflicts of interest to declare.
Please cite this article as: Fernández C, Calvo M, Leite N, López A, Ferreiro T, Ribera R, et al. Trasplante renal procedente de donante vivo HLA incompatible: Eficacia y pronóstico en 32 pacientes tras desensibilización. Nefrologia. 2017;37:638–645.