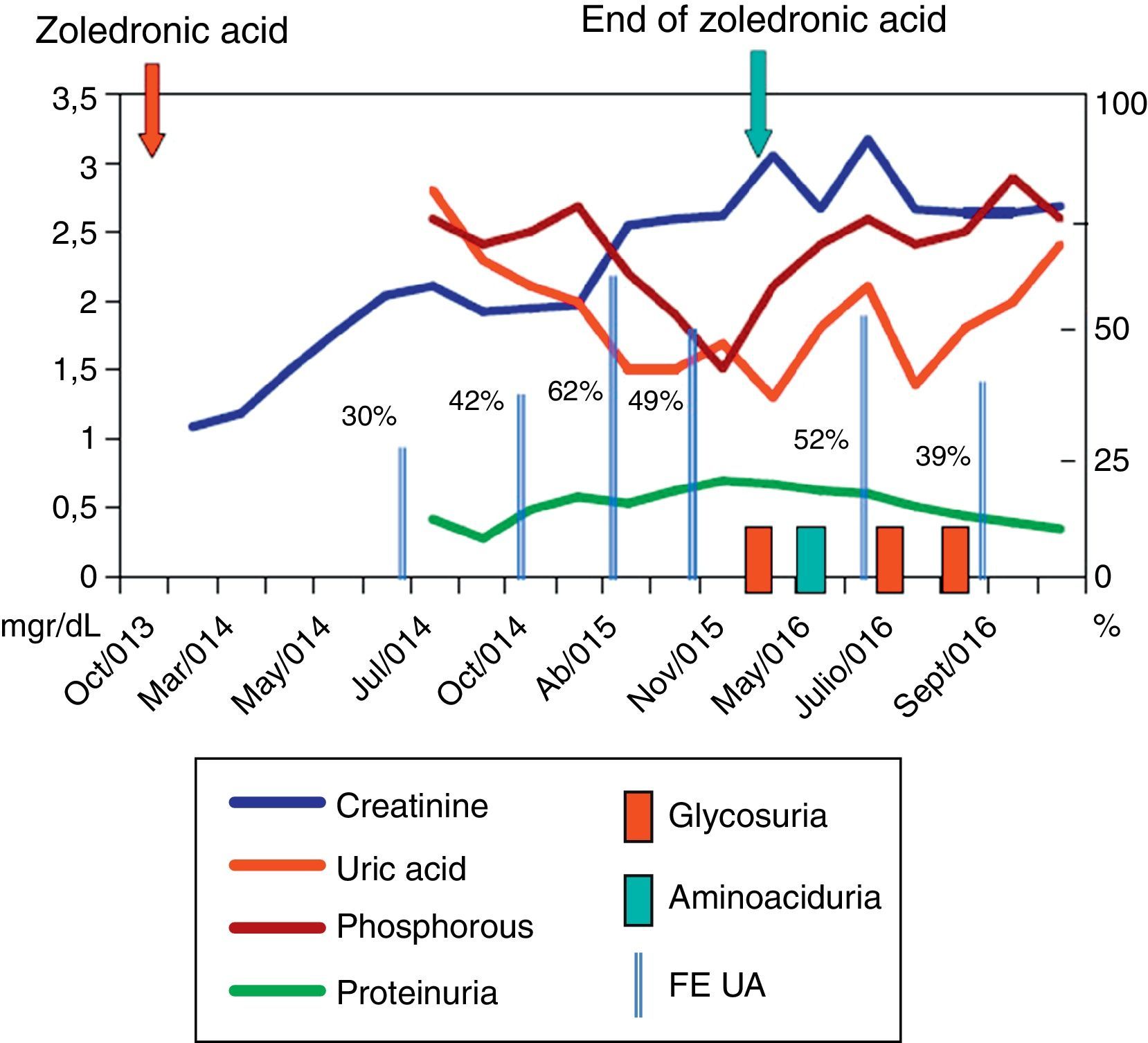
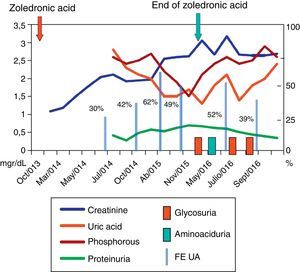
The relationship between kidney disease and bisphosphonates is illustrated in the medical literature1: the vast majority of cases involve glomerulonephritis and acute tubular necrosis. We discuss a case of tubulopathy related to the administration of zoledronic acid. This association has been described, although infrequently.
80-Year-old man with COPD and dyslipidaemia treated with inhaled indacaterol and aclidinium bromide, oral atorvastatin and omeprazole, who presented with acute kidney failure in October 2013 due to obstructive uropathy. He was diagnosed with Gleason score 10 prostate cancer and, after placement of urinary catheter, he recovered his baseline glomerular filtration rate (Cr: 1.1mg/dl). An extension study showed osteoblastic metastasis in the iliac wing. He was treated by a transurethral resection of the prostate and hormonal therapy (bicalutamide and leuprolide), to which intravenous zoledronic acid was added at a dosage of 4mg per month from November 2013. His glomerular filtration rate progressively deteriorated and he developed hypophosphataemia, hyperphosphaturia, hypouricaemia, hyperuricosuria, non-nephrotic range mixed proteinuria, metabolic acidosis with normal anion gap, and glycosuria with aminoaciduria, which was the reason for consultation. His accumulated dose of zoledronic acid was 116mg over 29 months. With these findings, he was diagnosed with kidney failure due to interstitial nephritis with Fanconi syndrome. The zoledronic acid was discontinued and substituted by denosumab. After 6 months of follow-up, the aminoaciduria and glycosuria disappeared, the uricosuria was reduced and the phosphataemia was normalised. Proteinuria continued to decrease, however, he only partially recovered glomerular filtration rate (Fig. 1).
Bisphosphonates are bene anti-reabsorbing agents used to treat corticosteroid-induced post-menopausal osteoporosis and to prevent pathological fractures in Paget's disease, prostate or lung cancer and multiple myeloma. Strong biphosphonates have a nitrogenous chain in their structure. They may be administered orally or intravenously. In the former, they do not tend to induce nephrotoxicity. Intravenous administration involves circumventing the hepatic metabolism, interaction with the P450 enzymatic system and its unaltered excretion in the kidney, through glomerular filtration and through active tubular secretion which would account for the tubular damage. The strongest are pamidronate and zoledronic acid. Both have been associated with focal segmental glomerulonephritis, with minimal changes, as well as with severe tubular damage. Zoledronic acid normally produces toxic acute tubular necrosis, in part due to by its long half-life in plasma (150–200 days) and low binding rate with plasma proteins (56%). In rats,2 a single dose of 1mg/kg induces proximal tubulopathy, while a dose of 10mg/kg provokes additional damage in the distal tubule. The ratio between the lowest lethal dosage and the minimal nephrotoxic dosage is 3.3.
In humans, it may cause direct mitochondrial damage and podocyte injury, secondary to farnesyl diphosphate inhibition, with decrease in prenylated proteins in the proximal tubule and direct toxicity to the parietal epithelial cells.
We have only found 3 cases of Fanconi syndrome described in the medical literature (PubMed dated 23 November 2016 with the keywords: Fanconi and zoledronic acid) attributed to zoledronic acid administration.
The first case3 is a 54-year-old woman with breast cancer who, 12 years after diagnosis, presented with hepatic and bone metastases treated with docetaxel, trastuzumab and zoledronic acid at doses of 4mg/month IV. After one year of treatment, she developed a proximal tubulopathy, with deterioration of glomerular filtration rate. Evidence of interstitial nephritis and tubulopathy were identified in the renal biopsy. After discontinuing the zoledronic acid, the glomerular filtration rate was recovered.
The second case4 is that of a 61-year-old woman with breast cancer and bone and liver metastases, which required zoledronic acid to be added to her basic treatment at a dose of 4mg/week. At the ninth week, (36mg accumulated) she developed proximal tubulopathy with glomerular filtration rate deterioration, which she also recovered upon discontinuing the zoledronic acid.
The third case5 is that of a 60-year-old woman with metastatic colon cancer undergoing treatment with FOLFOX-6 and bevacizumab together with zoledronic acid. She developed hypokalaemia, hypocalcaemia, hypophosphataemia and proximal tubulopathy, from which she recovered after zoledronic acid was discontinued and substituted by denosumab.
To the best our knowledge, this is the fourth reported case of Fanconi syndrome related to the administration of zoledronic acid. We believe it is important to bear in mind that interstitial nephropathy presenting with Fanconi syndrome is an undesirable effect of zoledronic acid which requires the close monitoring of tubular function in patients with this treatment.
Please cite this article as: Gutiérrez Sánchez MJ, Petkov Stoyanov V, Pedraza Cezón L, Martín Navarro JA. Insuficiencia renal por nefropatía tubulointersticial con tubulopatía proximal tipo Fanconi tras tratamiento con ácido zoledrónico. Nefrologia. 2017;37:660–661.