Venous aneurysms in the arteriovenous fistulas (AVF) are common, between 5% and 60% according to the series and the definition of aneurysm being used.1–3 In the majority of cases there is secondary weakness in the vessel wall due to repeated punctures. They are true dilations of the vessel, which conserves all its layers, unlike pseudoaneurysms, in which a rupture of the vascular wall.
There are circumstances that favor the development of aneurysms, such as vessel weakness associated with conditions such as Alport syndrome4 or polycystic kidney disease.5 The presence of proximal stenosis also fosters the onset and growth of aneurysms.6–8
The diagnosis of venous aneurysm is based on clinical findings, but conducting a Doppler ultrasound helps the diagnosis; it allows the measurement of the calibre and detects the presence of associated stenosis and intraluminal thrombus.
Juxta-anastomotic venous aneurysms (JVA) are rare (less than 2% of the total) and are distinguished from the rest by their pathogenesis and evolution.9,10
We present 4 cases of JVA treated with different surgical techniques since anatomical and clinical situations were different in each case.
They all grew and presented signs of cutaneous ischaemia.
Case 1: Radiocephalic AVF. Transplanted patient.
Treatment: JVA resection after fistula ligation, since access was not needed.
Case 2: Radiocephalic AVF. JVA and poor blood flow in dialysis sessions.
Treatment: new proximal anastomosis after aneurysm resection.
Case 3: Humerocephalic AVF.
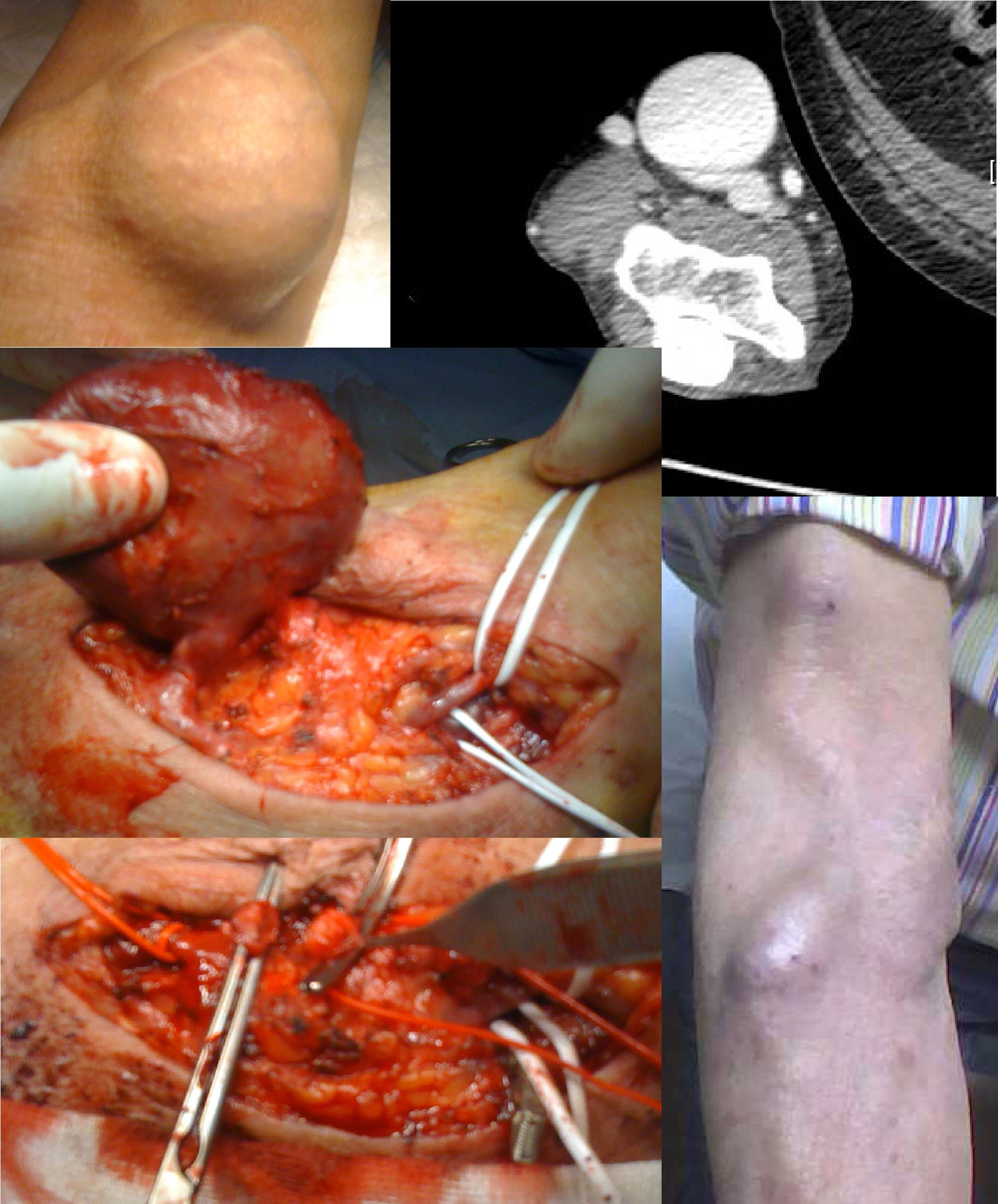
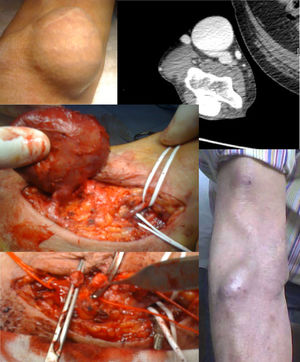
Treatment: JVA resection and reconstruction with median accessory cephalic vein (Fig. 1).
Case 4: Humerocephalic AVF.
Treatment: initially, angioplasty of a stenosis proximal to the aneurysm. The result was not satisfactory and it was decided a new intervention.
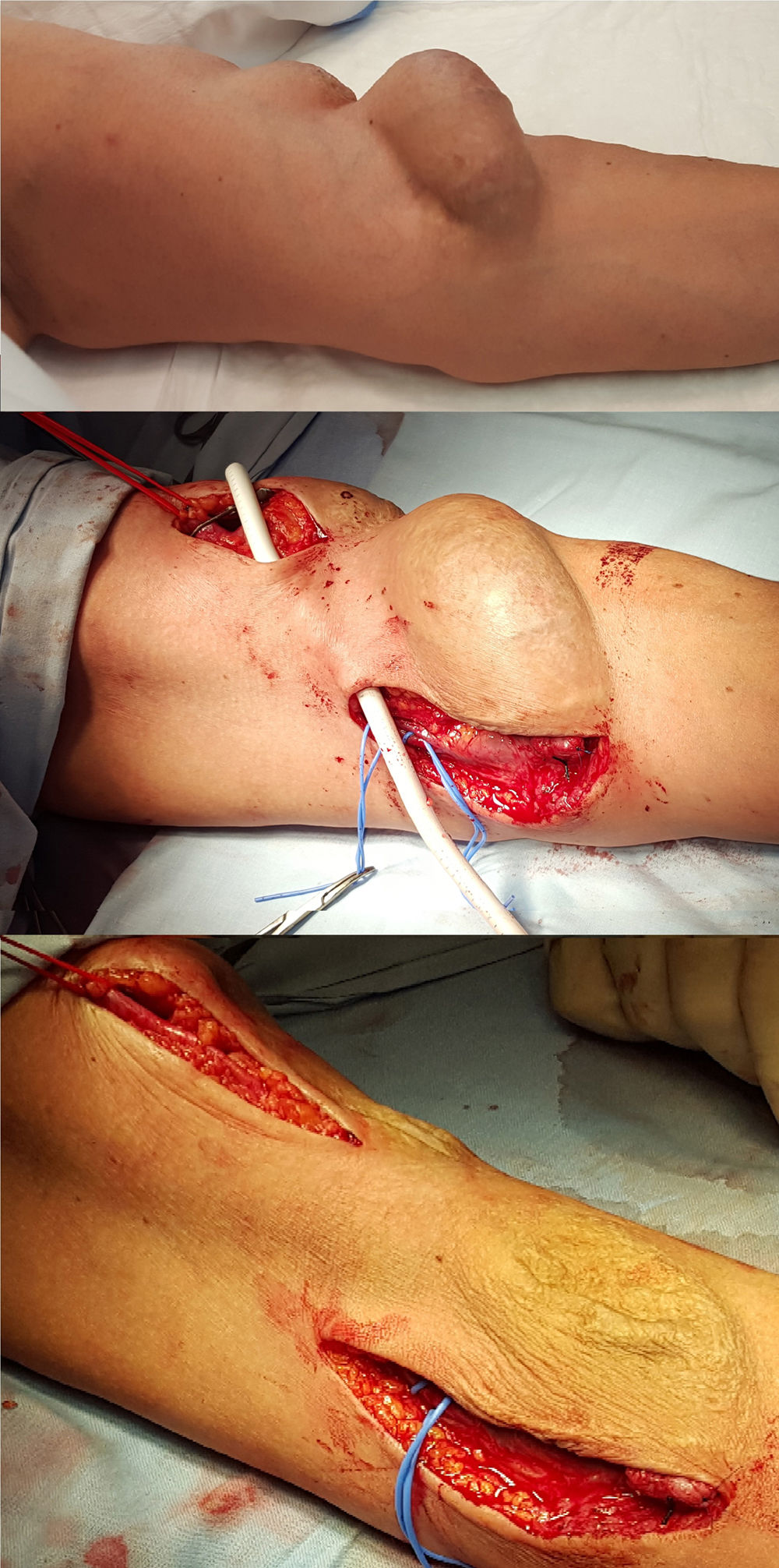
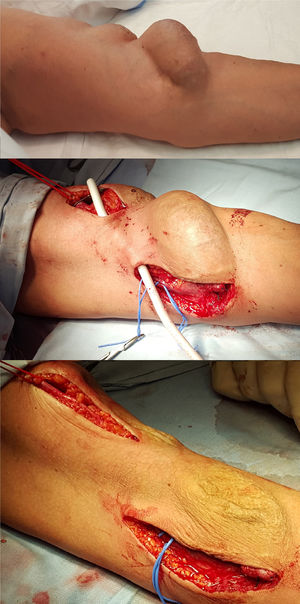
Autogenic reconstruction was not possible, the juxta-anastomotic aneurysm and another proximal one secondary to punctures was excluded by means of interposition of a PTFE-covered stent between the humeral artery and the proximal cephalic vein was ruled out (Fig. 2).
No patient presented post-operative complications.
It was possible to use all AVFs in the following dialysis session and they remain permeable, except the AVF that was ligated because he was a transplanted patient.
Treatment of venous aneurysms in AVFs is justified because it carries the risk of rupture and massive haemorrhage, which may cause death, excess of blood flow, pain due to compression of surrounding structures, epidermal necrosis, infection, stenosis due to partial thrombosis, inability to puncture the AVF, venous hypertension, or negative cosmetic effects.1
The various therapeutic options can be grouped into conservative, intravascular, or surgical treatment: exclusion with or without resection, aneurysmorrhaphy, with a possible need for a new autogenous or prosthetic AVF. In the event that the AVF is not going to be used, ligation is indicated.1
Endoprosthesis offers the advantage of correcting the stenosis associated with VA in the same procedure. But the subsequent punctures are more difficult and, occasionally, it still require aneurysmorrhaphy or simultaneous excision.1
The JVAs are rare and can be identified because they are not due to weakening of the vessel wall secondary to punctures.
The literature on this type of aneurysm and its treatment is scarce. Our experience differs from that published by Valenti et al., who advise an expectant attitude since growth of the aneurysm occurred and signs of cutaneous ischaemia appeared in all cases, which required intervention. This evolution seems logical, since the existence of proximal stenosis was demonstrated in all cases.
Angioplasty of the proximal stenosis was only conducted in case 4, to allow an expectant attitude, but a relapse of the stenosis occurred and the aneurysm grew up again. In case 3, an angio CT scan was necessary to analyze the anatomy of the aneurysm. Given the characteristics thereof, intervention by radiology was not advisable.
In the radiocephalic AVFs, it was decided to proceed with surgical treatment, in one case because the AVF was unnecessary and in the other case we followed our protocol that includes the performance of a new proximal anastomosis.
ConclusionsJVAs are rare and the pathogenesis is different to that of aneurysms secondary to puncture.
In our experience, they are associated with proximal stenosis, and they grow up and require treatment due to the risk of cutaneous ischaemia and rupture.
Please cite this article as: Jiménez-Almonacid P, Pila U, Gruss E, Lasala M, Rueda JA, Colás E, et al. Aneurismas venosos yuxtaanastomóticos en fístulas arteriovenosas para hemodiálisis. Nefrologia. 2018;38:454–457.