BK virus (BKV) is the main cause of nephropathy associated with polyomavirus (NAPV) in renal transplantation and is an important cause of graft loss. In recent years, polyomavirus nephropathy has been associated with JC virus (JCV),1 a less common entity with a later clinical presentation, which usually causes a delay in diagnosis. We present a case of nephropathy associated with JC polyomavirus (NAPV-JC) in a renal transplant patient, which is to our knowledge the first described in our country.
Our case is a 43-year-old male with a history of chronic kidney disease secondary to segmental and focal glomerulosclerosis on peritoneal dialysis since 2009, who received a deceased-donor donor kidney transplant in February 2011. He received basiliximab, steroids, tacrolimus and mycophenolate mofetil, and his maintenance therapy was: prednisone 5 mg/day, mycophenolate mofetil 2 g/day and tacrolimus (levels of 6−8 ng/dl).
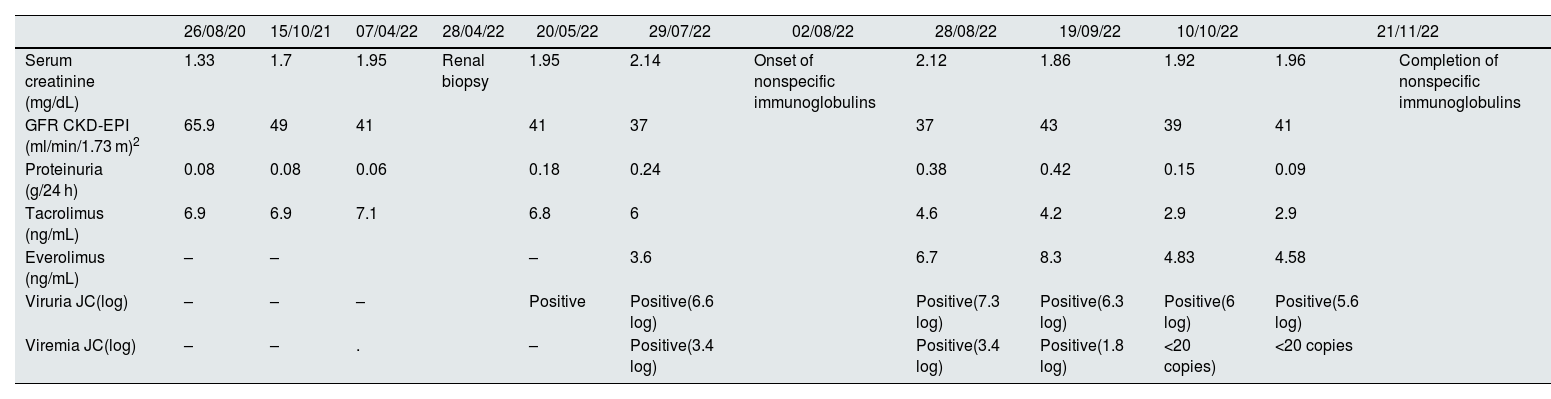
During 2021 the patient presented a progressive deterioration of renal function with serum creatinine levels of 1.95 mg/dl as compared with baseline serum creatinine of 1.2–1.3 mg/dl (Table 1). Microbiological study of plasma and urine ruled out cytomegalovirus and KBV infection. Measurement of anti-HLA antibodies and an ultrasound of the abdomen did not show data of interest. Therefore, it was decided to perform a renal biopsy in April 2022.
Evolution of analytical and microbiological results.
| 26/08/20 | 15/10/21 | 07/04/22 | 28/04/22 | 20/05/22 | 29/07/22 | 02/08/22 | 28/08/22 | 19/09/22 | 10/10/22 | 21/11/22 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serum creatinine (mg/dL) | 1.33 | 1.7 | 1.95 | Renal biopsy | 1.95 | 2.14 | Onset of nonspecific immunoglobulins | 2.12 | 1.86 | 1.92 | 1.96 | Completion of nonspecific immunoglobulins |
| GFR CKD-EPI (ml/min/1.73 m)2 | 65.9 | 49 | 41 | 41 | 37 | 37 | 43 | 39 | 41 | |||
| Proteinuria (g/24 h) | 0.08 | 0.08 | 0.06 | 0.18 | 0.24 | 0.38 | 0.42 | 0.15 | 0.09 | |||
| Tacrolimus (ng/mL) | 6.9 | 6.9 | 7.1 | 6.8 | 6 | 4.6 | 4.2 | 2.9 | 2.9 | |||
| Everolimus (ng/mL) | – | – | – | 3.6 | 6.7 | 8.3 | 4.83 | 4.58 | ||||
| Viruria JC(log) | – | – | – | Positive | Positive(6.6 log) | Positive(7.3 log) | Positive(6.3 log) | Positive(6 log) | Positive(5.6 log) | |||
| Viremia JC(log) | – | – | . | – | Positive(3.4 log) | Positive(3.4 log) | Positive(1.8 log) | <20 copies) | <20 copies | |||
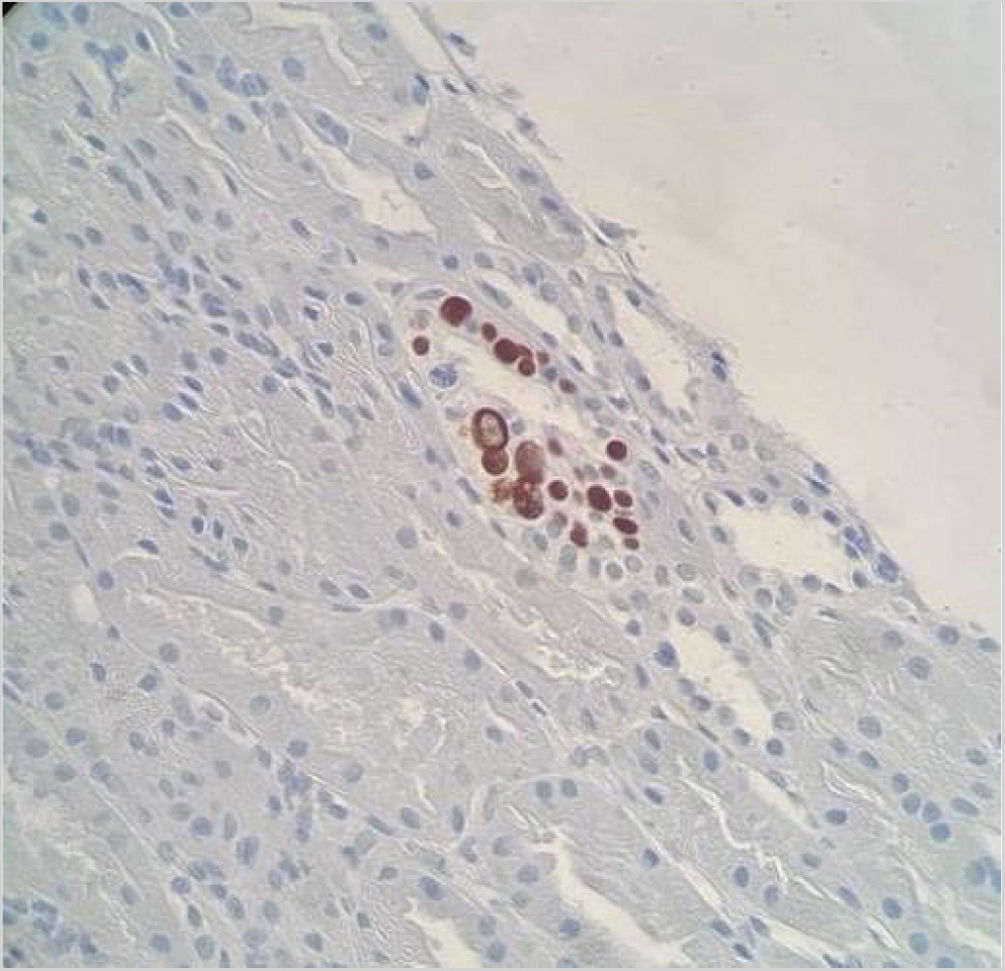
Renal biopsy showed a total of 18 glomeruli (seven with global sclerosis) and one medium caliber artery, mild chronic lymphoplasmacytic inflammation and areas of interstitial fibrosis and tubular atrophy. We observed numerous nuclear images compatible with viral inclusions, which were positive for SV-40 immunohistochemical staining (Fig. 1). The study with immunofluorescence and C4d staining was negative. The presumptive diagnosis was nephropathy associated with polyomavirus BK (NAPV-BK), however, the detection of BKV viral load in plasma and urine samples was repeatedly negative; therefore, we considered the involvement of another polyomavirus.
The presence of JCV was investigated by PCR (Primerdesign Poliomavirus JC-Genesig) in the renal biopsy sample previously deparaffinized and in urine and plasma samples, all of which were positive, establishing the diagnosis of NAPV-JC.
Mycophenolate mofetil was discontinued and everolimus was started while maintaining reduced tacrolimus levels. In addition, non-specific intravenous immunoglobulins were added as complementary treatment (30 g every 3 weeks, total 6 doses). The presence of JCV virus in urine and blood decreased progressively, with a more marked decrease of viremia, and stable serum creatinine levels without a significant proteinuria (Table 1).
JCV is an exclusively human pathogen, causing asymptomatic infection in healthy adults. Between 70 and 90% of the population is infected in childhood,1 producing a latent infection in the renal epithelium,2 from where it can be reactivated and excreted in the urine in both immunocompetent and immunocompromised individuals.3
NAPV-JC is a rare complication that occurs in renal transplant patients, with the first case described in 2003.4 Its incidence is low, up to 0.9%, unlike NAPV-BK whose incidence is higher (1–10%).5 However, it is possible that there is underdiagnosis, due to its atypical presentation and the high level of clinical suspicion required. Unlike NAPV-BK, which is usually observed within two years of transplantation, NAPV-VJC has a later onset, usually four years after graft implantation.4,6–8
Histological diagnosis of NAPV is based on the presence of tubulointerstitial inflammatory infiltrate and fibrosis of multifocal distribution associated with histological identification of polyomavirus from basophilic nuclear viral inclusions in epithelial cells and/or positivity for immunohistochemical staining for SV40 T antigen, which cross-reacts with BK and JC polyomaviruses. Therefore, the presence of the previous histological findings in the midst of viruria and negative viremia for BKV makes it necessary to rule out the presence of NAPV-JC, and JCV can be detected by PCR in urine, blood or previously deparaffinized renal biopsy samples.
There is no consensus about the treatment of NAPV-JC, with most clinicians using an approach like that for NAPV-BK given the virological similarity, and the main strategy is reduction of immunosuppression. Other therapeutic agents such as intravenous immunoglobulins, leflunomide or conversion to m-TOR inhibitor (i-mTOR) have also been used with variable results.6,7
In summary, NAPV-JC is an infrequent condition that requires a high clinical suspicion for its diagnosis, given its histological similarity to NAPV and negative virological tests for VBK. The main therapeutic measure consists of reducing immunosuppression; however, given the small number of published cases, more studies are needed to learn and establish more standardized management guidelines for this condition.