To the Editor,
Different scientific societies have frequently revised the recommendations for the treatment of anaemia in patients with pre-dialysis (no-D) chronic kidney disease (CKD), which include target values of haemoglobin (Hb), ferritin and transferrin saturation rate (TSAT). In 2005, the Agence Française de Sécurité Sanitaire des Produits de Santé recommended target Hb values between 110-130g/l.1 The KDOQI2006 guidelines (Kidney Disease Outcomes Quality Initiative) recommend not intentionally maintaining Hb>130g/l and in 2007, recommended a range of 110-120g/l.2 In 2007, the European Medicine Agency recommended an Hb range of 100-120g/l and in 2009, the ERBP guidelines (European Renal Best Practice) recommended 110-120g/l, not exceeding the value of 130g/l.3,4 The latest update to the KDOQI2012 guidelines recommends maintaining an Hb level between 100-115 g/l.5
With the aim of evaluating the degree of compliance of the established target values according to the different guidelines, we carried out a retrospective observational study of all the patients with no-D CKD and non-transplant patients treated with an erythopoiesis-stimulating agent (ESA) on 1 September 2012, with no changes made to the dosage for a minimum of 6 months. We obtained our data from the outpatient pharmacy programme and the hospital clinical workstation. The following variables were recorded: age, sex, levels of Hb, ferritin and TSAT, CKD stage, type of ESA and monthly dose, treatment concurrent with angiotensin-converting-enzyme inhibitors (ACE inhibitors), angiotensin II receptor antagonists (ARBs), iron supplements and treatment for secondary hyperparathyroidism (SHPT); and diabetes mellitus (DM) as underlying comorbidity.
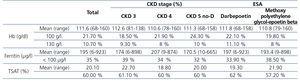
The study included a total of 305 patients (141 male) with stage 3 CKD (65 patients), stage 4 CKD (140 patients) or no-D stage 5 CKD (100 patients). The mean age was 68 years (range: 22-95 years). The administered average monthly dose of ESA (expressed in micrograms of darbepoetin) was 90µg. 17% of the patients were being treated with ARBs and 14 % with ACE inhibitors. 40 % took iron supplements and 60 % received treatment for SHPT. 25% of patients presented DM.
Mean Hb was 112g/l, mean ferritin 195µg/l and mean TSAT 20% (Table 1). Statistically significant differences were not observed between the parameters studied (mean Hb, mean ferritin, mean TSAT, Hb<100g/l, Hb>130g/l, ferritin <100µg/l and TSAT<20%) according to the CKD stage, the type and dose of ESA, the iron supplement, the concomitant medication for anaemia (ACE inhibitors, ARBs), SHPT controlled with drugs or DM. There are a higher percentage of patients with Hb<100g/l in more advanced stages of CKD.
The degree of compliance of the established objectives according to different guidelines is shown in Figure 1. Mainly, the percentage within the suitable range is less than 60%. Only 40.7% of the patients presented an Hb level within the range recommended by the KDOQI2012 guidelines, and 54.7 % according to the objectives of the ERBP. The results of this study coincide with those from a study by OCEANE, in which only 50% of the patients met the objective values of Hb (100-120g/l).6 In another previous study, the percentage of patients compliant with the objective Hb values (100-120g/l) was 38.4%. According to Valderrabano et al., 96% of the patients had levels of Hb>100g/l.8 In our results this percentage was 78.3%. These results reflect the difficulty in maintaining Hb within the recommended margins, due to a narrow objective range, the variability of Hb values and the complexity of patients (age and cardiovascular comorbidities).
Conflicts of interest
Dr A. Martinez-Castelao has collaborated with Novartis, Boëhringer, Abbvie, Shire, Amgen and Roche laboratories and has participated in the advisory boards of the laboratories Abbvie and Amgen.
Table 1. Values of haemoglobin, ferritin and the rate of transferrin saturation according to the stage of renal function and the type of erythropoiesis-stimulating agent.
Figure 1. Degree of compliance of the established objectives according to the different clinical guidelines.