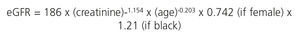La enfermedad renal crónica (ERC) es un importante problema de salud pública que puede afectar en sus diferentes estadios a cerca del 10 % de la población española y que supone una elevada morbilidad y mortalidad, así como un importante consumo de recursos al Sistema Nacional de Salud. Diez sociedades científicas involucradas en el manejo del paciente renal nos hemos puesto de acuerdo para hacer una puesta al día del anterior documento de consenso sobre ERC de 2007. El presente es la edición abreviada del documento general extenso, que puede ser consultado en las páginas web de cada una de las sociedades firmantes. Contiene los siguientes aspectos: definición, epidemiología y factores de riesgo de la ERC. Criterios de diagnóstico, evaluación y estadiaje de la ERC, albuminuria y estimación del filtrado glomerular. Concepto y factores de progresión. Criterios de derivación a Nefrología. Seguimiento del paciente, actitudes y objetivos por especialidad. Prevención de la nefrotoxicidad. Detección del daño cardiovascular. Actitudes, estilo de vida y tratamiento: manejo de la hipertensión arterial, dislipemia, hiperglucemia, tabaquismo, obesidad, hiperuricemia, anemia, alteraciones del metabolismo mineral y óseo. Seguimiento coordinado por Atención Primaria-otras especialidades-Nefrología. Manejo del paciente en tratamiento renal sustitutivo, hemodiálisis, diálisis peritoneal y trasplante renal. Tratamiento paliativo de la uremia terminal. Esperamos que sirva de gran ayuda en el manejo multidisciplinar del paciente con ERC, a la vista de las recomendaciones más actualizadas.
Chronic kidney disease (CKD) is a major public health problem that, in its different stages, may affect up to 10% of the Spanish population and results in high morbidity and mortality, as well as high consumption of National Health System resources. Ten scientific societies involved in the management of kidney patients agreed to update the 2007 CKD consensus document. The current version is an abridged edition of the detailed general document, which can be consulted on the webpages of each signatory society. It includes the following aspects: CKD definition, epidemiology and risk factors and criteria on diagnosis, assessment and staging of CKD, albuminuria and glomerular filtration estimation. Progression factors and concept. Criteria for referral to Nephrology. Patient follow-up, attitudes and objectives by specialty. Prevention of nephrotoxicity. Detection of cardiovascular damage. Attitudes, lifestyle and treatment: management of high blood pressure, dyslipidaemia, hyperglycaemia, smoking, obesity, hyperuricaemia, anaemia and mineral and bone metabolism disorders. Coordinated follow-up by Primary Care – other specialties – Nephrology. Management of renal replacement therapy, haemodialysis, peritoneal dialysis and renal transplantation patients. Palliative treatment of terminal uraemia. We hope that this document will be very useful in the multidisciplinary management of CKD patients, in view of the updated recommendations.
INTRODUCTION
Chronic kidney disease (CKD) is a major public health problem. According to the results of the EPIRCE (Epidemiology of Chronic Renal Failure in Spain) study, designed to obtain information about the prevalence of CKD in Spain, promoted by the Spanish Society of Nephrology (S.E.N.) with the support of the Ministry of Health and Consumption, 9.24% of the adult population has some degree of CKD.1 6.83% of the population has a glomerular filtration rate (GFR) of less than 60ml/min/1.73m2, with this percentage being 20.6% in those over 64 years of age. As well as high prevalence, CKD is associated with high cardiovascular morbidity and mortality and very significant costs. In Spain, the annual cost of treatment in the most advanced stages of CKD is estimated to be more than 800 million euros.2
Conveying in one document both CKD detection strategies and risk situations in which there is a high likelihood of progression to end-stage renal disease or higher morbidity and mortality will undoubtedly help identify individuals who are at higher risk of renal progression or cardiovascular complications at an early stage. Likewise, establishing strategies for preventing and managing CKD and its complications by Primary Care, as well as criteria for the appropriate referral of patients to Nephrology comprise the aspects covered in this document. Its purpose is, therefore, to prevent, detect, refer to the specialist and manage CKD, with the aim of improving kidney health and prognosis in our patients.
This consensus document arises from the need to revise and update the previous document developed in 2007 by S.E.N.-SEMFyC (Spanish Society of Family and Community Medicine) on CKD,3 after an extensive review of the most recent literature and the latest clinical practice recommendations. The methodology used herein was based on a critical review of the main clinical guidelines on CKD and on the occasional support of the few randomised studies in CKD patients.
We considered it appropriate to involve in its drafting scientific societies whose objectives include care for kidney patients and, we therefore developed the consensus between the ten signatory societies. Each of them appointed its representatives (who appear as authors) in the drafting of the document, which was subsequently submitted for approval by their respective boards of directors. The document was displayed on the webpages of ten societies and was open to possible suggestions by their members. These suggestions were incorporated into the final complete text and were also sent for display on the respective webpages of the signatory societies.
This document is an abridged summary of the original document.
CHRONIC KIDNEY DISEASE: DEFINITION AND EPIDEMIOLOGY
All of the guidelines consulted,4,5 including the current 2012 KDIGO (Kidney Disease Improving Global Outcomes) guidelines, published in January 2013,6 confirmed the definition of CKD (independently of the clinical diagnosis) as the presence, for at least THREE MONTHS, of:
- an eGFR (estimated glomerular filtration rate) less than 60ml/min/1.73m2.
- Or renal lesion.
Renal lesion may be revealed directly from histological abnormalities in the renal biopsy or indirectly through the presence of albuminuria or urinary sediment abnormalities or by using imaging techniques.
In Spain, it is estimated that 9.24% of the adult population suffers from some degree of CKD, with 6.83% of the general population having stages 3-5 CKD. CKD prevalence is increasing due to an ageing population, the increased prevalence of risk factors such as cardiovascular disease and diabetes mellitus (DM), high blood pressure (HBP) or obesity and, obviously, due to its early diagnosis. It is estimated that renal replacement therapy consumes 2.5% of the National Health System’s budget and more than 4% of that of Specialist Care.2
Chronic kidney disease risk factors
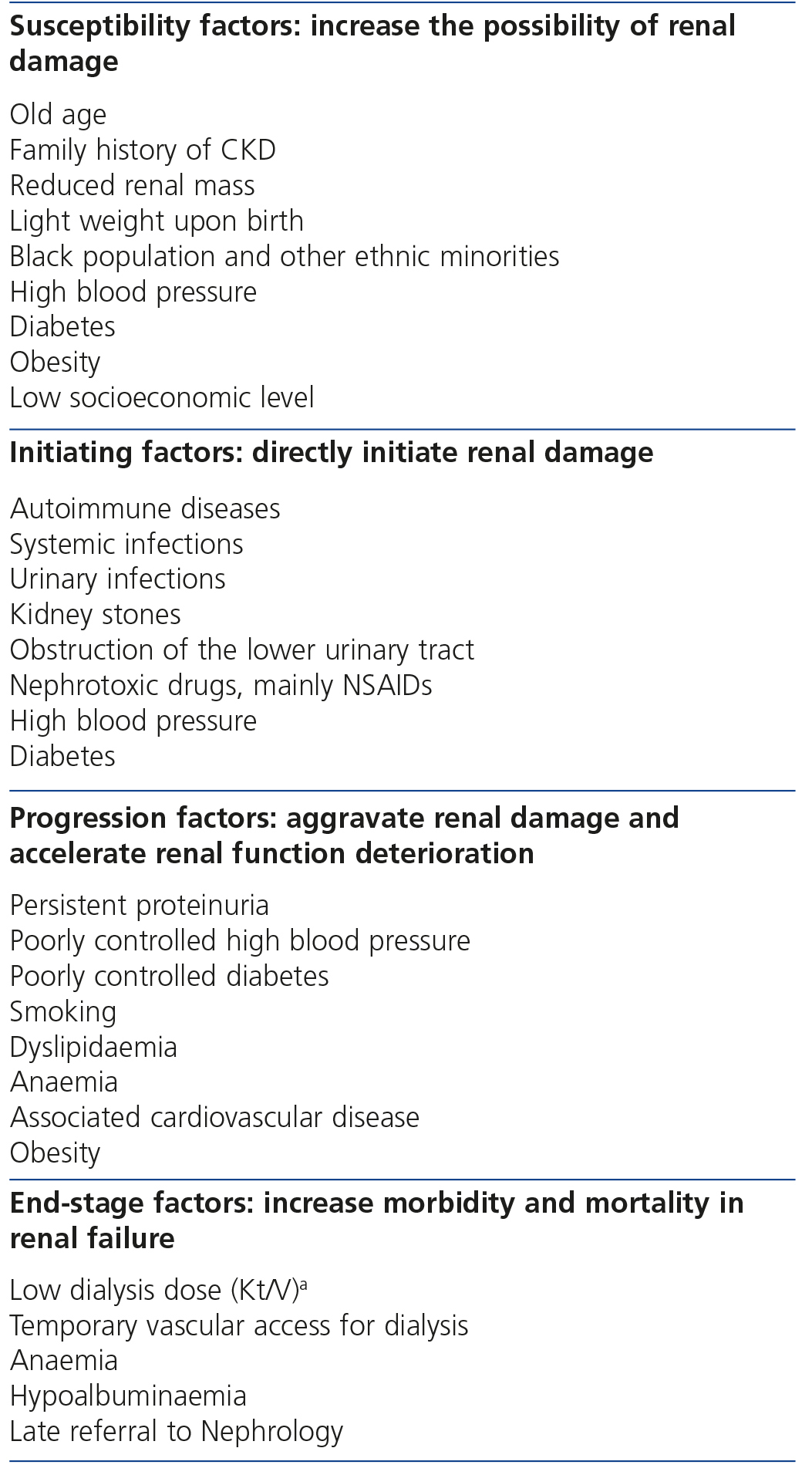
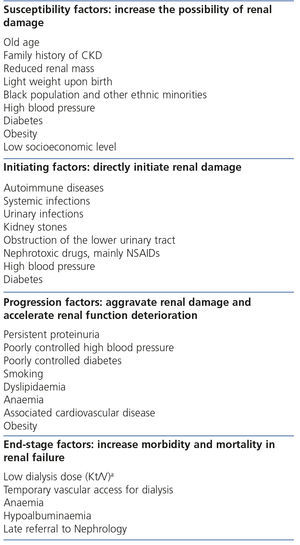
The continuous conceptual CKD model7 includes risk factors for each phase, which are classified into susceptibility, initiating, progression and end-stage factors (Table 1). Some risk factors may be included in the susceptibility, initiating and progression factor categories, such as HBP.
Chronic kidney disease screening
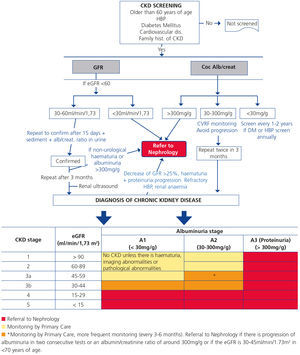
CKD screening in risk populations should be carried out by assessing the eGFR and albuminuria at least once a year. The diagnosis must not be based on one SINGLE eGFR and/or albuminuria test and must ALWAYS be confirmed.
We recommend CKD screening in patients with HBP, type 2 DM or established cardiovascular disease. Likewise, its screening is advised in individuals older than 60 years of age, those who are obese (body mass index [BMI] >35kg/m2), with type 1 DM for more than five years, patients with first degree relatives who have kidney disease or hereditary kidney diseases, patients with urinary tract obstruction diseases, patients on prolonged treatment with nephrotoxic drugs (including non-steroidal anti-inflammatory drugs [NSAIDs]), patients with other cardiovascular disease risk factors (hyperlipidaemia, metabolic syndrome, smokers), those with a history of acute renal failure and those with chronic infections, autoimmune diseases and neoplasia associated with CKD.
DIAGNOSIS OF CHRONIC KIDNEY DISEASE
Estimation of the glomerular filtration rate
Serum creatinine concentration should not be used as the only test for assessing renal function, with GFR being the best tool for doing so. Calculating the GFR from creatinine clearance (measurement of creatinine concentration in serum and 24-hour urine) has a series of disadvantages, such as overestimation of the GFR and the problem posed by collecting 24-hour urine both for the patient and for laboratories. Measuring creatinine clearance by collecting 24-hour urine does not improve GFR estimation from equations except in certain circumstances.
We recommend estimating the GFR using equations obtained by measuring serum creatinine concentration, age, sex and ethnicity. These equations are more accurate than an isolated measurement of serum creatinine. The equations most used are those of the Modification of Diet in Renal Disease study (MDRD-4 or MDRD-IDMS),8 in accordance with whether the method used by the laboratory to measure serum creatinine is traceable or not (Table 2) in the isotope dilution mass spectrometry (IDMS) reference measurement procedure, with the latter being recommended.
The CKD-EPI (Chronic Kidney Disease-Epidemiology Collaboration) equation,9 also using standardised creatinine methods, has advantages over MDRD-IDMS, given that it is more accurate and improves the predictive capacity of the GFR (particularly between values of 60 and 90ml/min/1.73m2), as well as improving the prediction of overall and cardiovascular mortality and the risk of developing ESRD.10 As such, it is considered that CKD-EPI should replace the previous formulas (Table 3). The new 2012 KDIGO guidelines consider the use of alternative formulas to be acceptable if they have been shown to improve accuracy when compared with the CKD-EPI formula.6
This equation has demonstrated that it is superior to other GFR estimation equations based on the serum concentration of creatinine (MDRD), cystatin C or the combination of both.10-13
Although the Cockcroft-Gault (C-G) equation14 has classically been used in the adjustment of drug doses and has been a reference for assessing hyperfiltration states, it should be advised against. This equation has not been reformulated for creatinine values obtained using appropriate procedures and cannot be re-expressed for the current methods of measuring creatinine, and as such, it should not be used. The CKD-EPI or MDRD-IDMS equations may be used for this purpose, since they are based on standardised creatinine measurement procedures. The GFR obtained by MDRD or CKD-EPI is useful for drug dose adjustment, since it correlates better than that obtained by C-G for values below 60ml/min/1.73m2, which are the most likely to require a dose adjustment and are available in the clinical laboratory reports, unlike C-G.10,11,15,16
The values obtained using the MDRD or CKD-EPI equations are adjusted for a body surface area (BSA) of 1.73m2. However, when there is a need to use the formula or adjust particularly toxic drugs or those with a low therapeutic index in patients with major deviations in BSA, GFR values should not be standardised to 1.73m2. In these cases, it is only necessary to multiply the laboratory result expressed in ml/min/1.73m2 by the patient’s real BSA ratio divided by 1.73m2 (GFR x BSA/1.73m2).
In general, the use of equations for estimating the GFR (MDRD and CKD-EPI) is inadequate in a series of clinical situations, especially in individuals with an extreme body weight (BMI <19kg/m2 or 35kg/m2), those with special diets or malnutrition, muscle mass disorders, amputations, patients <18 years of age, those with liver disease, pregnant women, individuals with acute renal failure and in the study of potential kidney donors. In these cases, for an adequate measurement of renal function, we would require 24-hour urine collection in order to calculate renal clearance.3
Until present, no clinical practice guidelines have included the use of cystatin C or the GFR estimated from it as a CKD screening parameter, but the new 2012 KDIGO guidelines6 suggest measuring cystatin C in adults with a GFR between 45 and 59ml/min/1.73m2, without other renal lesion markers, if diagnostic confirmation of CKD is required. The recently published CKD-EPI equation for cystatin C should therefore be used.
Assessment of renal lesion
Albuminuria (urinary excretion of albumin)
Albuminuria is, along with GFR, the basis for CKD diagnosis and staging. The persistent presence of high protein or albumin concentrations in urine is not only a sign of renal lesion, but is also often a sign of “systemic damage” beyond the kidney. Different studies have demonstrated the importance of proteinuria in the pathogeny of CKD progression, as well as the link between albuminuria and renal prognosis and mortality in various populations independently of the GFR and other classic cardiovascular disease risk factors.
We recommend not using terms such as micro- or macroalbuminuria and employing the term albuminuria or urinary albumin excretion and the absolute value of the albumin/creatinine ratio (ACR) in urine, preferably in the first urine of the morning. The ACR is a more sensitive marker than proteinuria in the context of CKD secondary to DM, HBP or glomerular disease, which are more common causes of CKD in adults.
In the case of patients with CKD diagnosis and significant proteinuria (for example, an ACR >300-500mg/g), monitoring could be carried out using the protein/creatinine ratio in urine since it is a more economical test and because, as proteinuria increases, particularly in nephrotic proteinuria, the ACR is less sensitive. The use of the protein/creatinine ratio in urine is also recommended in patients who have suspected interstitial disease and nephrotoxicity due to antiretrovirals, since in both situations, proteinuria mainly involves low molecular weight proteins that are different from albumin. In order to determine albuminuria in a patient, two high values in three samples obtained over a 3 to 6 month period are necessary.
The value and persistence of albuminuria are closely related to renal and survival prognosis in CKD patients, but we must also consider that albuminuria is a major independent marker of overall cardiovascular risk (endothelial dysfunction, arterial remodelling), and not only of chronic kidney disease. The presence of albuminuria alone, without any other sign of renal damage, is called into question by various authors as being specific to CKD, since it may be detected in other diseases (e.g. obesity, smoking, dermatitis and arthritis).
We must remember that determining proteinuria includes not only the quantification of albuminuria, but also the quantification of low molecular weight proteins, such as proteins of tubular origin or immunoglobulin light chains.
Urinary sediment abnormalities
The presence of haematuria and/or leukocyturia in the urinary sediment for more than three months, once a urological cause or urinary infection have been ruled out (including urinary tuberculosis), may also be an indication of CKD.
X-ray pathological images
Renal ultrasound firstly allows to rule out the presence of an obstructive urinary tract pathology, but also to identifiy structural abnormalities that indicate the presence of renal damage. Simple isolated renal cysts alone are NOT criteria for renal damage.
Histological abnormalities
The indication of biopsies is part of the specialist field in Nephrology.
New chronic kidney disease staging
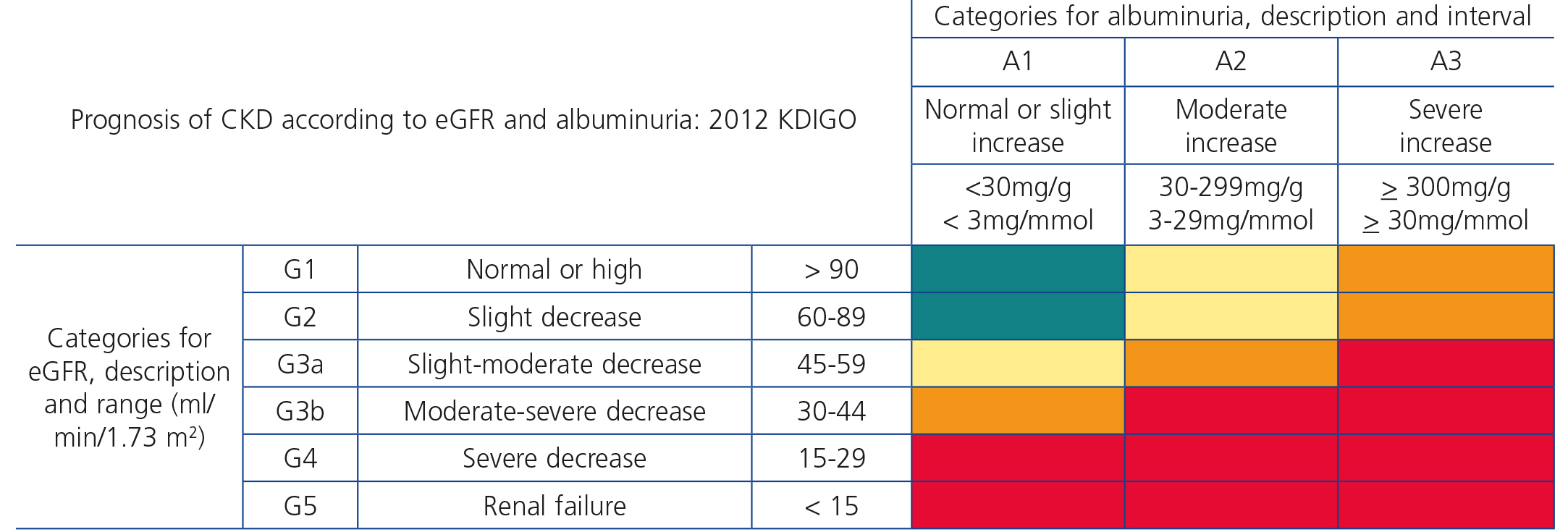
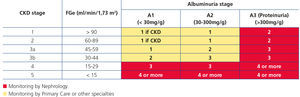
Recently, from the results of different clinical studies that include normal individuals, those at risk of developing CKD and CKD patients, the international organisation KDIGO6 has established a new CKD prognostic classification based on eGFR and albuminuria stages. This classification is divided into six risk categories according to the eGFR (G1-G5), which include three risk categories according to ACR concentration: A1 for optimal or normal-high values (<30mg/g or <3mg/mmol), A2 for moderately high values (30-299mg/g or 3-29mg/mmol), and A3 for very high values (≥300mg/g or ≥30mg/mmol), respectively (Table 4).
DEFINITION OF CHRONIC KIDNEY DISEASE PROGRESSION
The mean annual GFR decrease is very variable and is higher in patients with significant proteinuria, DM or HBP.
Key points
1. Normal renal progression rate: 0.7-1ml/min/1.73m2 per year from 40 years of age.6
2. We can consider that a patient has renal progression: with a GFR >5ml/min/year or >10ml/min in five years.1
3. Progression must be defined on the basis of two aspects:
- Progression to a higher renal function deterioration category (<30, 30-299, >300mg/g).
- Percentage of change with respect to the baseline situation (>25% deterioration in the GFR) or a more than 50% increase in the ACR.
4. For assessing renal progression, we recommend albuminuria and baseline GFR estimation, as well as the identification of renal progression factors. This will indicate the frequency of successive laboratory tests.
5. To ensure the accuracy of renal deterioration rate measurement, the aforementioned guidelines advise carrying out two measurements of eGFR within a period of no less than three months and ruling out a decrease due to acute renal failure or the start of treatment with drugs that affect glomerular haemodynamics (angiotensin converting enzyme inhibitors [ACEI], angiotensin II receptor blockers [ARBs], NSAIDs, diuretics).
6. In patients with a new diagnosis of CKD (for the first time), GFR estimation must be repeated within a period of no less than three months, in order to rule out acute renal deterioration due to exogenous factors (diarrhoea, vomiting, depletion due to diuretics or any drug that affects glomerular haemodynamics, such as ACEI, ARBs or direct renin inhibitors). It may be repeated within less than three months where the clinical situation indicates. In patients with known CKD, it is suggested to measure the eGFR and the ACR once a year if they have a low risk of progression, and more frequently if there is a high risk of progression.
PREDICTORS OF PROGRESSION
The factors of renal progression are displayed in Table 5.5,17-28
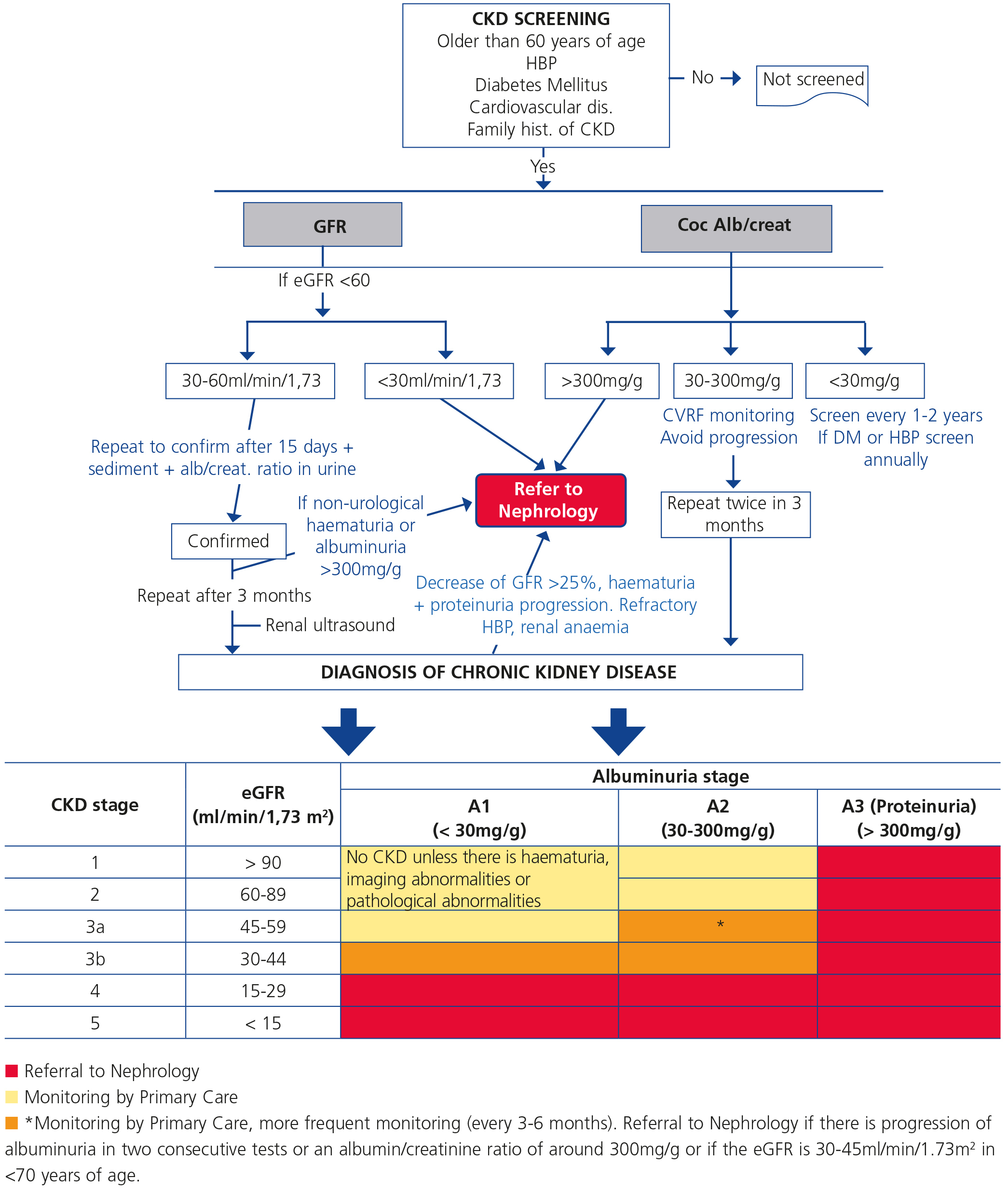
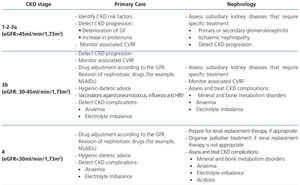
CRITERIA FOR REFERRAL TO NEPHROLOGY (Figure 1)
Referral to Nephrology will be carried out taking into account the CKD stage, the speed of renal failure progression, the degree of albuminuria, warning signs, associated comorbidities and the patient’s functional situation.3,5,29
Generally speaking, patients who have an eGFR <30ml/min/1.73m2 should be referred to the Nephrology specialist (except those >80 years of age without renal progression and albuminuria <300mg/g).
According to glomerular filtration rate:
- All patients with an eGFR <30ml/min/1.73m2, except patients >80 years of age without renal progression.
- Patients >80 years of age and with an eGFR <20ml/min/1.73m2, if their general condition advises it, can be referred for a nephrological assessment and agree on treatment. It is recommended for the candidate patient to be referred to Nephrology at least one year prior to the start of renal replacement therapy. Although this period is not easy to calculate, renal progression (see point 5) may be used as a guideline. The objective is to avoid a patient who is a candidate for renal replacement therapy requiring non-scheduled dialysis.
Patients <70 years of age with an eGFR between 30 and 45ml/min/1.73m2 should be monitored more frequently (every 3-6 months) and only be referred to Nephrology in the case of albuminuria progression in two consecutive tests or when the ACR ratio is around 300mg/g.
- According to albuminuria: ACR ratio >300mg/g, equivalent to proteinuria >300mg/24 hours.
Other reasons:
- Acute deterioration of renal function (>25% decrease in the eGFR) in less than a month, having excluded exogenous factors (diarrhoea, vomiting, depletion due to diuretics during treatment with ACEI, ARBs or direct renin inhibitors).
- Patients who have renal progression (>5ml/min/year) (see definition above).
- CKD and HBP refractory to treatment (>140/90mmHg) with three drugs at full dose, one of them being a diuretic.
- Abnormalities in potassium (>5.5mEq/l or <3.5mEq/l without the patient receiving diuretics).
- Anaemia: haemoglobin [Hb] <10.5g/dl with CKD in spite of correcting iron deficiency (transferrin saturation index [TSI] >20% and ferritin >100).
- Warning signs:
- Non-urological haematuria associated with proteinuria.
- Decrease of >25% in the eGFR in less than one month or a >25% increase in plasma creatinine in less than one month, ruling out exogenous factors (diarrhoea, vomiting, depletion due to diuretics during treatment with ACEI, ARBs or direct renin inhibitors).
There may be Primary Care or joint follow-up, according to the cases.
Elderly patients (>80 years of age)
Given that CKD progression is very uncommon in the elderly population, we can accept that patients older than 80 years of age with stable renal function or with a slow deterioration of the latter (<5ml/min/year) without proteinuria or anaemia or warning signs can be followed up conservatively in Primary Care.30,31
Likewise, elderly patients with stage 5 CKD who are not expected to live for long (<6 months), who have a poor functional situation (dependence in daily activities, dementia, etc.) or severe associated comorbidity or who do not accept dialysis may be suitable for palliative treatment either in Primary Care or shared with Nephrology.32
Diabetic patients
Patients will be referred to Nephrology with the aforementioned criteria in mind, and furthermore, the following patients must be referred:
- Those with albuminuria: a (confirmed) ACR >300mg/g, despite suitable treatment and blood pressure (BP) monitoring.
- An increase in albuminuria despite suitable treatment.
- Refractory HBP (three drugs at full dose and absence of control).
Indications for a request for ultrasound in Primary Care
Either for patient follow-up in Primary Care or for their referral to Nephrology, the request for an ultrasound in the diagnostic study of CKD is considered to be relevant. It is indicated in:
- Progressive CKD (decrease in the eGFR >5ml/min/1.73m2 in one year).
- Macroscopic haematuria or persistent albuminuria.
- Symptoms of urinary tract obstruction.
- Age >15 years and a family history of polycystic kidney disease.
- Stage 4 or 5. Assess associated comorbidities beforehand.
- CKD with proteinuria.
- Recurrent urinary infections with renal involvement.
MONITORING AND FOLLOW-UP OF CHRONIC KIDNEY DISEASE PATIENTS
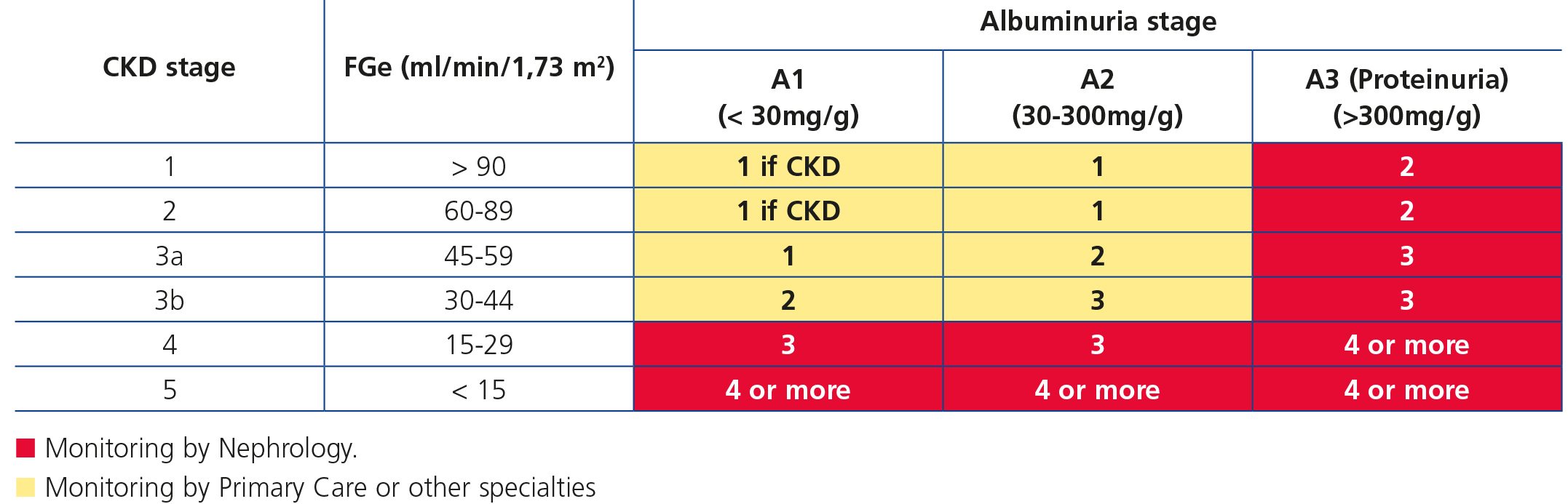
The frequency of monitoring and visits of CKD patients is displayed in Table 6. In any case, it is necessary to individualise these general criteria.
For each check-up in Primary Care, we recommend:
• Monitoring BP and adjusting treatment. BP objective <140/90mmHg. In patients with proteinuria (ACR >300mg/g), we recommend around 130/80mmHg. In elderly patients, this measurement will be carefully individualised.33 Avoid low blood pressure in elderly patients with significant atheromatous disease.
• Monitoring anaemia: if the patient has stage 3-5 CKD and Hb <10.5g/dl (once iron deficiency has been ruled out: TSI >20% and ferritin >100ng/ml), estimate referral or advance it in Nephrology in order to assess treatment with erythropoiesis-stimulating factors.
• Reviewing medication and adjusting the dose in accordance with the GFR. In stages 3-5 CKD, avoid using NSAIDs, oral anti-diabetic drugs eliminated in the kidneys and iodinated contrasts.
• Reviewing dietary habits and guiding the patient on the type of diet to follow according to the GFR:
- Stages 1-3 CKD: a low sodium diet is only recommended in the case of HBP.
- Stages 4-5 CKD: dietary recommendations for sodium, phosphorus and potassium.
• Laboratory tests in each check-up from stage 3 CKD* (the minimum advised is in bold):
- Complete blood count.
- Blood biochemical analysis: glucose, creatinine, urea, Na, K, Ca, P, albumin and cholesterol. GFR estimated using MDRD or CKD-EPI.
- Urinary biochemical analysis (single urine sample, first in the morning): ACR.
- Urinary sediment.
The extractions should be combined so that they do not have to be repeated. The patient will be provided with a report or, failing that, a copy of the tests. If the Nephrology check-ups are carried out once a month, it is not necessary to repeat the tests in Primary Care.
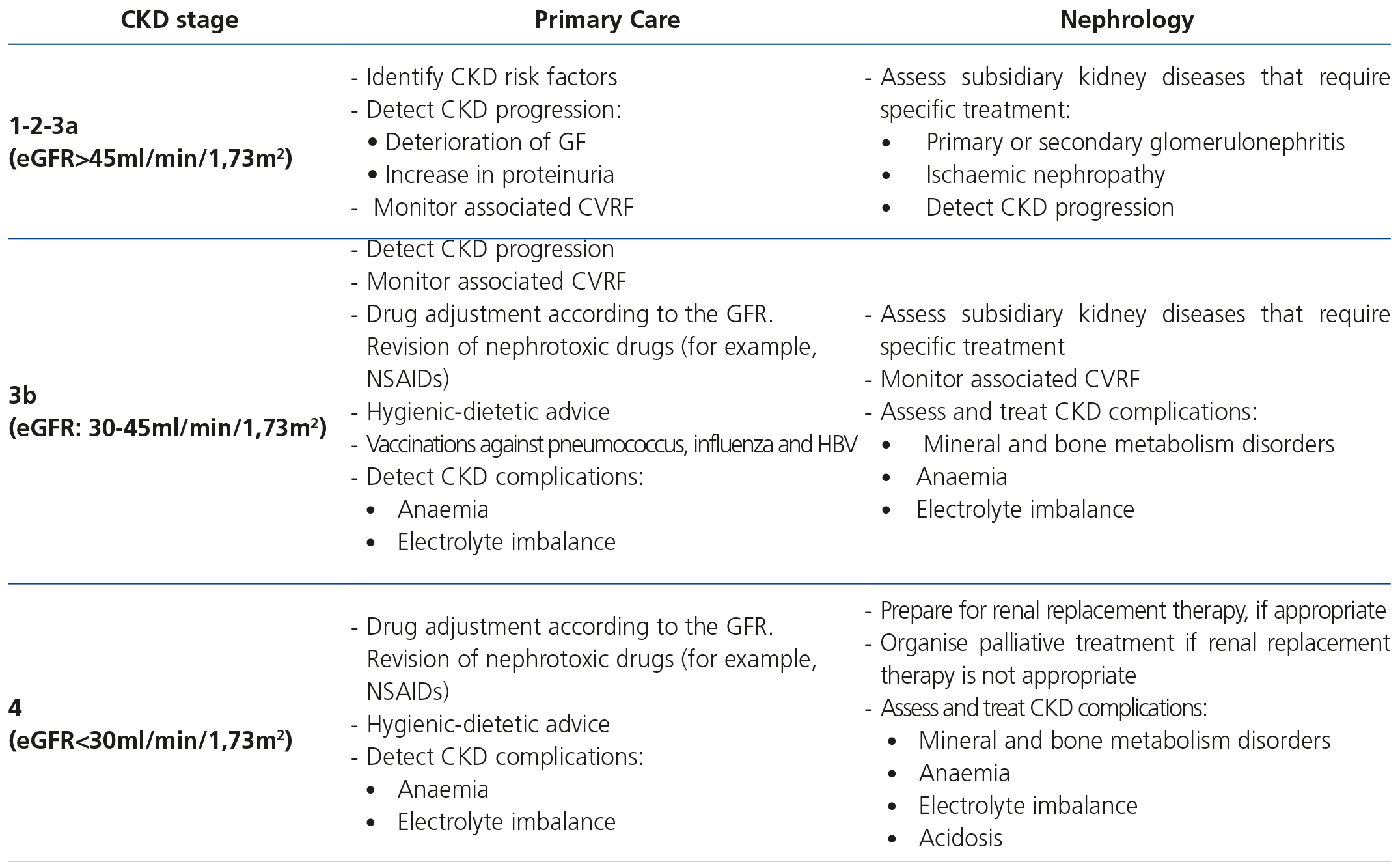
Table 7 displays the objectives of monitoring and follow-up of CKD patients according to the stage.
PREVENTION OF NEPHROTOXICITY
Once the patient has been diagnosed with CKD, the most important thing is to avoid its evolution, and as such, we must be aware that there are drugs used in daily practice, and more specifically, in these patients, that may cause this disease to worsen. Moreover, the indiscriminate use of intravenous contrast agents without prior preparation may cause contrast-induced nephropathy. As such, it is necessary to avoid using nephrotoxins and minimise the effect and use of intravenous contrasts.
Avoid nephrotoxins and take precautions with drugs that may alter glomerular haemodynamics
1. Avoid hyperkalaemia associated with drugs. Particular caution must be taken with the association of a potassium-sparing diuretic (spironolactone, amiloride, eplerenone) to another potassium-retaining drug (ACEI, ARBs, direct renin inhibitors, NSAIDs, beta blockers). In these cases, frequent monitoring of serum potassium is essential.
2. Avoid using drugs that may cause rapid volume depletion and tubular damage, particularly in situations of dehydration, as well as those with a direct negative effect on the tubule (aminoglycosides, tacrolimus, cyclosporin A).
3. We must particularly emphasise avoiding the unnecessary use of NSAIDs, due to the risk of renal function deterioration.
4. Adjust drugs to the GFR, particularly in elderly and diabetic individuals. In these patients, metformin (it should not be administered when the eGFR is <30ml/min/1.73m2), oral anti-diabetic drugs eliminated via the kidney, new anticoagulants, nephrotoxic antibiotics and some heparins must be used with caution. Many drugs produce direct nephrotoxicity and their detrimental effect worsens when combined with those that interfere with glomerular haemodynamics. It is very important to avoid them in risk situations or adjust their dose in accordance with the eGFR (aminoglycosides, vancomycin, aciclovir, tenofovir, amphotericin, etc.). The list of drugs for adjusting the dose can be consulted on the following webpage: http://nefrologiadigital.revistanefrologia.com/modules.php?name=libro&op=viewCap&idpublication=1&idedition=13&idcapitulo=109.
Minimise the use of intravenous contrasts
Contrast-induced nephropathy is defined as a deterioration in renal function manifested as a relative 25% increase in creatinine or an absolute increase in creatinine of 0.5mg/dl with respect to baseline, which occurs during the first three days following administration of the contrast, not due to any other mechanism. It occurs due to direct toxicity in tubular cells.34
The most important aspect for preventing nephropathy by contrast is to detect it in patients at risk of developing it (old age, heart failure, DM, previous renal failure, dehydration, acute myocardial infarction, shock, contrast volume, anaemia, low blood pressure, the use of nephrotoxins and high doses of diuretics, taking care with drugs that alter glomerular haemodynamics and avoiding low blood pressure). The best treatment is prevention, avoiding risk situations. We recommend discontinuing diuretics at least 4-6 days before administering the contrast, as well as correct hydration using intravenous fluid therapy and oral hydration. Some drugs may be potentially toxic after contrast administration, such as metformin. However, there is currently not sufficient evidence to discontinue metformin in patients with previously normal renal function who are administered a “moderate” amount of contrast,35 although some authors suggest this.
ATTITUDES, LIFESTYLE AND TREATMENT
Physical exercise
As a general rule, it is recommended to carry out 30-60 minutes of moderate exercise 4 to 7 days a week. When the kidney disease is established, the exercise must be adapted to the physical ability of each patient.
Diet
Dietary recommendations must be individualised in order to avoid patients being overweight or obese, but also in accordance with their renal function and the existence of other risk factors in which some specific restriction is indicated.
• Stages 1-3 CKD: low salt diets are only recommended for HBP and/or heart failure.
• Stages 4-5 CKD: dietary recommendations on sodium, phosphorus, potassium and proteins.
The energy requirements are similar to those of the general population. The information available suggests that protein restriction delays progression of kidney failure and should start to be applied when the GFR falls below 30ml/min, except in cases of proteinuria due to hyperfiltration, where it must be established long before, even with normal renal function. Protein content must be adjusted to 0.8g/kg/day (at least half must be animal proteins of a high biological value), but with a fat-based high-calorie content (mono and polyunsaturated) and carbohydrates, if there is not carbohydrate intolerance or dyslipidaemia that require additional adjustments. The appropriate protein intake in CKD patients is 0.8g/kg, 50% of which must be of high biological value, that is, of animal origin; the remaining 50% must be complemented with proteins contained in the rest of the foods included in the diet (vegetable origin). The use of high-protein diets, as well as drugs that cause weight reduction, may have adverse effects in CKD.36
The daily consumption of salt must be less than 6g (the equivalent of 2.4g of sodium). In initial phases of kidney disease, a stricter salt restriction will only be applied to patients with HBP. The diet must be complemented with potassium and phosphorus restriction and vitamin D intake.
In CKD patients on haemodialysis (HD), protein intake may be increased by up to 1.2g/kg in order to achieve an adequate protein balance, avoid calorie-energy exhaustion and attain an adequate nutritional status.
Alcohol
Moderate alcohol intake is not considered to be harmful, as with the general population, that is, around 12 to 14g of alcohol (around 300cc of beer or 150cc of wine). But we must bear in mind not only the calories present in alcohol, but also the quantity of liquid and the sugar, potassium, phosphorus and sodium content, which must be limited in many patients in accordance with associated risk factors and the degree of renal failure that they may cause.
SPECIFIC OBJECTIVES OF TREATMENT
High blood pressure in chronic kidney disease: treatment objectives5,33,37
• In CKD patients, the objective of anti-hypertensive treatment is threefold: to reduce BP, to reduce the risk of cardiovascular complications and to slow down CKD progression.
• In patients with CKD and an ACR <30mg/g, the recommended BP control target is ≤140/90mmHg. If the ACR is ≥30mg/g, a stricter target is suggested, with BP ≤130/80mmHg, both in patients with and those without DM.
• The drugs of first choice are those that block the renin-angiotensin system actions, either ACEI or ARBs.
• The use of anti-hypertensive drug combinations is recommended to achieve the control objectives. This combination must include a thiazide or loop diuretic depending on the severity of CKD.
Hyperglycaemia management in chronic kidney disease
Management objectives
How is metabolic control assessed?
Glycated haemoglobin A1C (HbA1C) is the reference parameter for assessing metabolic control in patients with chronic renal failure (CRF) and we must bear in mind factors that limit its usefulness as a glycaemic control marker, such as transfusions and treatment with erythropoietin.38-43
Objectives of glycaemic control
The guidelines recommend that in patients with short-term DM and without a decrease in life expectancy, the target must be HbA1C <7 (<53mmol/mol).44
By contrast, for patients with long-term DM with a previous history of poor glycaemic control or with a condition that decreases life expectancy, the control target must be individualised, avoiding therapeutic strategies that involve an unacceptable increase in the risk of hypoglycaemia.
There is no evidence that indicate what the optimal HbA1C level is for dialysis patients.
It is necessary to bear in mind that the risk of severe hypoglycaemia in patients with renal failure on intensive treatment is very high and is increased by a decreased intake, a change in meal times and the presence of autonomic neuropathy.
Oral anti-diabetic drugs44
Secretagogues
Sulfonylureas (SU) are not the first choice drug in renal failure.
Glibenclamide and glimepiride are metabolised in the liver to weaker metabolites, but are eliminated in urine, and as such, even in low doses, their use is not recommended in patients with CRF.
Glipizide is metabolised to inactive metabolites and, consequently, it would be the only SU that could be administered in CRF, but its use is not permitted with a low GFR (CCr <30ml/m).
Repaglinide is metabolised in the liver and less than 10% is eliminated via the kidneys. Nevertheless, a low dose of treatment (0.5mg) should initially be administered.
Metformin
Metformin is mainly eliminated in urine without being metabolised. In its data sheet, its use is not recommended with an eGFR <60ml/min, but this recommendation is not usually followed in normal clinical practice. With an eGFR <45ml/min, it is recommended to monitor glycaemia and renal function frequently and not administer it with an eGFR <30ml/min.
Alpha-glucosidase inhibitors
Both acarbose and miglitol and their metabolites accumulate in renal failure and their use is therefore not recommended.45
Glitazones
Glitazones are metabolised in the liver and less than 2% is eliminated in urine. There is therefore no accumulation of active metabolites in renal failure. However, given that their use increases the risk of oedema, heart failure and osteoporosis, it is limited in these patients and contraindicated in those on dialysis.
Dipeptidyl peptidase-4 inhibitors
When there is an eGFR greater than 50ml/min/1.73m2, no gliptine requires adjustment. Sitagliptin, vildagliptin and saxagliptin require a dose adjustment whenever the GFR is less than 50ml/minute.
Sitagliptin must be used at doses of 50mg and 25mg whenever the GFR is between 50 and 30ml/minute and less than 30ml/minute, respectively.
Vildagliptin must be used at doses of 50mg whenever the GFR is less than 50ml/minute, including in ESRD requiring dialysis.
Saxagliptin must be used at doses of 2.5mg in patients with a GFR of less than 50ml/minute. Saxagliptin is not indicated in patients with ESRD or those on dialysis.
Linagliptin does not require dose adjustment in any stage of CKD.
Glucagon-like peptide-1 analogues
Liraglutide is only indicated in patients with an eGFR >60ml/min/1.73m2. Experience with liraglutide and exenatide in this field is very limited.
Insulin
Insulin requirements are very variable and as such, individualisation of treatment is essential. We can indicate the following initial rules that must be adapted to each patient through glucose control:
• CCr >50: does not require a dose adjustment.
• CCr 50-10: will require a 75% reduction of the previous insulin dose.
• CCr <10: will require a 50% reduction of the previous insulin dose.
The insulin regimen will be adapted to the control target and may be a conventional therapy or an intensive treatment, although we should remember that the basal-bolus regimen has a lower rate of hypoglycaemia.
Objectives and management of dyslipidaemia
One of the factors that increase renal damage and accelerate renal function deterioration is dyslipidaemia, independently of its arteriosclerosis-promoting effect.
Risk stratification
In accordance with the latest European guidelines, CKD individuals must be considered to be patients with high or very high cardiovascular risk, without the need to apply risk scales. As such, CKD patients with a GFR <60ml/min/1.73m2 are classified as having a very high cardiovascular risk.46
Dyslipidaemia screening must be performed systematically. Although LDL (low-density lipoprotein) cholesterol is the main risk predictor, non-HDL (high-density lipoprotein) cholesterol may be better, as is the case in diabetics or metabolic syndrome patients.
Evidence on the benefit of treating dyslipidaemia on chronic kidney disease
Data obtained by post hoc analysis support the ability of statins to reduce cardiovascular complications in patients with stages 2 and 3 CKD.47,48
The results in stages 4 and 5 or in HD are not as clear.49,50 However, in the SHARP (Study of Heart and Renal Protection) study, a 17% reduction in cardiovascular events was observed in patients with stages 3, 4 and 5 CKD treated with simvastatin-ezetimibe compared with placebo.51 This reduction was not observed in patients treated with dialysis.
Lifestyle recommendations
Diet is the main determinant of cholesterol levels. The basic advice is therefore dietary. It is recommended that 30% or less of the total calories come from fatty foods and that less than 10% be saturated fats. It is advised that no more than 300mg/day of cholesterol be consumed.
Lipid-lowering drugs and chronic kidney disease
Statins
It is not necessary to adjust statin dose, except in very advanced stages of CKD3-5 and only for those that are eliminated via the kidney. They are the treatment of choice. CKD, as well as old age, female sex, a low BMI, liver dysfunction, alcohol consumption, systemic diseases and hypothyroidism, increase the risk of side effects, however, these are not common.
Drugs that are eliminated via the liver are the drugs of choice (fluvastatin, atorvastatin, pitavastatin and ezetimibe). Statins metabolised via CYP3A4 (atorvastatin, lovastatin, simvastatin) may increase side effects by enhancing pharmacological interactions when they are administered with certain inducing drugs (phenytoin, phenobarbital, barbiturates, rifampicin, dexamethasone, cyclophosphamide, carbamazepine, omeprazole) or inhibitors.
For kidney transplant patients, certain interactions must be borne in mind, particularly with cyclosporine and statins, such as atorvastatin, lovastatin and simvastatin, since they may increase their levels and the risk of myopathy. Fluvastatin, pravastatin, pitavastatin and rosuvastatin are less likely to interact. Although tacrolimus is also metabolised by CYP3A4, it seems that there is less risk of it interacting with statins. In these patients, statins must be introduced at low doses, titrated with caution and the interactions must be monitored.
Fibrates
Most guidelines recommend gemfibrozil as the fibrate of choice and they recommend avoiding the others. The risk of myopathy increases more than five times when it is combined with a statin and this is greater in CKD. The association with statins may cause acute renal failure due to rhabdomyolysis. If this combination is necessary, fenofibrate must be used and strict monitoring must be carried out. The normal dose of gemfibrozil is 600mg/day and it can be used in patients with a GFR of between 15 and 59ml/min. Its use is not advised if the GFR is <15ml/min. However, given the lack of evidence on the cardiovascular benefit of hypertriglyceridaemia treatment with fibrates and their potential side effects, treatment with fibrates is not recommended in CKD, particularly in combination with statins.
Ezetimibe
Its efficacy along with simvastatin has been demonstrated in CKD patients in the SHARP study.51 A dose adjustment is not necessary for renal failure.
Control objectives
CKD is a coronary heart disease risk equivalent; therefore, the objectives are the same as in patients with ischaemic heart disease.
The therapeutic objective in CKD patients (GFR <60ml/m) is LDL cholesterol <70mg/dl or a 50% reduction if the previous objective is not achievable.16 However, recently the KDIGO guidelines for treating dyslipidaemia in CKD recommend therapy with statins for all adults >50 years of age, independently of LDL cholesterol levels. Based on the SHARP study and in the post hoc analysis of clinical trials with statins compared with a placebo that studied CKD patients, a strategy for “treating cardiovascular risk” has been established. Likewise, initiating treatment with statins in stage 5 CKD patients not on dialysis is not recommended.52
After the creation of this document, the new international guidelines on dyslipidaemia management in CKD (Kidney Int Suppl 2013; 3(3): 259-305) were published, which suggest not pursuing objectives, but rather a strategy of “action”.
Smoking
Smoking is one of the factors directly involved in kidney disease progression and data have been published that link this habit to renal function deterioration in the general population (the MRFIT study,53 the study by Briganti et al.56) and in diabetics.57-60
Therefore, in all patients with CKD (as with the general population), we must ask patients about their tobacco consumption in all consultations that we perform (both in primary and in specialised care). Empathetic but firm and motivated advice should be given to smokers to encourage them to quit smoking, and emphasis must be put on the individual benefits and the help available to achieve it (systematic minimal intervention, cognitive-behavioural techniques, pharmacological treatment, etc.).
In kidney disease patients, nicotine replacement therapy (patches, chewing gum, sweets) seems to be safe, as well as their combination with lower doses than normal of bupropion (150mg/24h) in advanced stages of the disease. Varenicline use at normal doses seems safe and, as with bupropion, it can be used at half the dose (1mg/24h) of that of patients with moderately decreased renal function and in the general population.
Obesity
There are few specially designed clinical trials in this regard, but there are data that support that the reduction of weight and intake of fat may decrease the risk of CKD.61
Treatment of obesity in CKD patients must be non-pharmacological and consist of physical exercise and a low-calorie diet, in accordance with the recommendations of the corresponding section of these guidelines.
The only drug authorised in Spain for treating obesity, orlistat, is indicated in individuals with a BMI higher than 30kg/m2. It has interactions with many drugs and has not been studied in CKD patients, and as such, its use does not appear to be advisable in them. The use of drugs to reduce appetite is not indicated in CKD patients.
Hyperuricaemia
Hyperuricaemia is defined as the increase of uric acid levels above their solubility level in plasma. This occurs in males with uric acid values greater than 7mg/dl and in females, due to the oestrogenic effect, with values greater than 6mg/dl. Hyperuricaemia may be asymptomatic or cause diseases such as uric nephrolithiasis, nephropathy due to uric acid, tophaceous gout, acute gouty arthritis and asymptomatic hyperuricaemia. An increased cardiovascular risk has been reported with uric acid values in the high normal limit, above 5.2mg/dl.62,63
It has been demonstrated that allopurinol and other xanthine oxidase inhibitors have effects on the circulatory system that are independent of uric acid concentration.64-66
The clinical guidelines do not recommend treating asymptomatic hyperuricaemia, since this would only be supported by two randomised clinical trials.67,68 Another drug, febuxostat, has recently become available for treating hyperuricaemia in patients with a history of gout or uric arthritis. In those with symptomatic hyperuricaemia and mild to moderate renal failure, febuxostat administration has demonstrated greater efficacy and a similar safety to allopurinol, without the need to adjust the dose.69
Colchicine is indicated for treating acute gout attacks. In patients with a GFR between 30 and 50ml/min, the dose must be reduced. If the GFR is less than 30ml/min/1.73m2, colchicine use is contraindicated. In the event of an acute gout crisis in these patients with a reduced eGFR, intramuscular tetracosactrin (Nuvacthen depot®) may be administered for three days or corticosteroids in doses of 2-30mg/day with a rapid reduction until they are discontinued after 5-7 days.
Anaemia
The main cause of anaemia in CKD patients is the inadequate production of endogenous erythropoietin, a hormone that acts on the differentiation and maturation of red blood cell precursors. In CKD patients, anaemia is defined as a situation in which Hb concentration in blood is two standard deviations below the mean Hb concentration of the general population, corrected for age and sex.70 The lower limit of Hb levels from which anaemia is considered to exist in females is 11.5g/l,71 according to the S.E.N. and 12g/l according to the World Health Organization (WHO), the Kidney Disease Outcomes Quality Initiative (KDOQI) and the European Renal Best Practice (ERBP).72 The lower limit of Hb values in males younger than 70 according to the S.E.N., KDOQI and ERBP is 13.5g/l and 13g/l according to the WHO. For males older than 70 years of age, the S.E.N. and the WHO set the lower Hb limits at 12g/l and the KDOQI and ERBP set it at 13.5g/l.
Diagnosis of anaemia and iron and erythropoiesis-stimulating agent administration assessment and criteria
Characteristics of anaemia in chronic kidney disease
Anaemia associated with CKD is usually normocytic and normochromic in origin and is related to a decrease in erythropoietin production by peritubular cells, low bone marrow response, an increased production of hepcidin and a decrease in the availability of iron for erythropoiesis.73
When to start the study of anaemia in chronic kidney disease
• When Hb is <11g/dl in premenopausal females and prepubertal patients.
• When Hb is <12g/dl in adult males and postmenopausal females.
How to study anaemia in chronic kidney disease, laboratory requests
• Haematocrit (Ht)-Hb.
• Haemocytometer readings: mean corpuscular volume (MCV), mean corpuscular Hb (MCH), mean corpuscular Hb concentration (MCHC).
Reticulocytes.
• Iron parameters: serum iron, ferritin, transferrin, TSI.
• Rule out intestinal blood losses (if microcytic hypochromic anaemia or suspicion of digestive bleeding).
• In patients with stage 5 CKD on HD, samples are taken immediately before dialysis.
Haemoglobin targets
In adult CKD patients, Hb control targets between 10 and 12g/dl must be aimed for and symptoms and comorbidity must be assessed. If in patients with stages 3B to 5 CKD Hb <10.5g/dl is found, they should be referred to Nephrology if they were not being followed-up or they should be reviewed at an early stage.
Iron metabolism required before the start of treatment with erythropoiesis-stimulating agents
Sufficient caution should be taken to achieve and maintain target Ht/Hb:
• TSI ≥20% and <50%.
During treatment with erythropoiesis-stimulating agents (ESA), iron metabolism should be studied every three months, if the patient receives intravenous Fe.
In patients with ESA without intravenous Fe, the monitoring should be monthly until Hb has stabilised between 10 and 12g/dl.
In diabetic patients, it is recommended to not start treatment with ESA until Hb <10g/dl has been achieved, if the patient has a history of stroke.
In patients not treated with EPO, the target must be TSI ≥20% and Ferritin ≥100ng/ml. Patients must be monitored every 3-6 months.
The test must be carried out 15 days after the last dose of intravenous Fe is administered.
Intravenous iron administration regimen
It is administered with the purpose of preventing deficiency and maintaining iron reserves in order that the target Ht/Hb is achieved and maintained. The administration must be carried out in the hospital. With certain joint protocols, some intravenous Fe may be administered in the health centre under medical supervision.
Oral iron administration regimen
• Adults: 200mg/day.
• Children: 2-3mg/kg/day.
In pre-dialysis, home dialysis and PD adults who do not achieve adequate iron reserves with Fe by the oral route, a 100mg infusion of Fe dextran or 500-1000mg of intravenous Fe carboxymaltose should be administered and this process should be repeated as necessary in accordance with the iron parameters. Another option is intravenous iron sucrose (maximum 200mg/dose).
It is considered unlikely for HD patients to achieve the target with oral Fe, and as such, intravenous iron will be required.
Route of erythropoiesis-stimulating agent administration
Subcutaneous administration is indicated in HD, PD and home dialysis patients, with the injection location being rotated.
The intraperitoneal route would be possible when doses are administered to the empty abdomen or with a low amount of peritoneal fluid. Higher doses may be required using this route.
The intravenous route would be indicated in the case of high doses (volume) or recurrent ecchymosis at the site of injection.
EPO dose and adjustment
It is prescribed by Nephrology services.
Transfusions in chronic kidney disease patients
• In patients with functional anaemia.
• In patients who are resistant to EPO with chronic blood loss.
Potential adverse side effects of treatment with erythropoiesis-stimulating agents
HBP, seizures, arteriovenous fistula thrombosis, increase in blood viscosity. Treatment with ESA when there is Hb >13g/dl has been associated with high levels of cardiovascular disease, although without an increase in mortality.74
Anaemia control in CKD patients must incorporate educational programmes for patients that include information about the health issue, professional support and lifestyle guidance.
Detection of mineral and bone metabolism disorders
Calcium and phosphorus metabolism disorders in CKD are associated with various complications that are beyond simple bone involvement and include other systems, particularly the cardiovascular system (for example, calcifications). The earliest clinical manifestation is an increase in the parathyroid hormone (PTH), caused by active vitamin D deficiency (calcitriol), phosphate retention (with or without hyperphosphataemia) and/or marked hyperphosphataemia.
Detection and treatment objectives
• Avoid hyperphosphataemia.
• Maintain normal levels of calcium and phosphorus.
• Avoid the onset and progression of secondary hyperparathyroidism.
According to the 2003 K-DOQI, 2007 S.E.N. and 2011 S.E.N. guidelines,75-77 the therapeutic objective is variable in different stages of CKD, but it may be summarised by saying that we should aim to maintain calcium, phosphate and PTH within the normal limits. In stage 4, it is even advised to maintain PTH values slightly higher than normal values. The guidelines also recommend measuring calcidiol (25-OH-vitamin D) in order to diagnose the deficiency or insufficiency of vitamin D. These levels should ideally be greater than 20-30ng/m (50-75nmol/l).
A small degree of stable hyperparathyroidism is not a concern, but progressive hyperparathyroidism, with PTH values that are two or three times higher than the reference value, require consultation with Nephrology specialists. Nephrologists should also be consulted for patients with high levels of phosphate that are greater than 5mg/dl (1.40mmol/l).
Treatment
Treatment will be carried out through diet, phosphate binders, native or active vitamin D, and/or selective activation of vitamin D receptors. Calcimimetics may be used in dialysis patients.
Drugs for adequate maintenance of mineral metabolism
Phosphate binders
These are administered with meals. Binders with calcium include calcium carbonate, calcium acetate or its combination with magnesium. Binders without calcium or aluminium include sevelamer and lanthanum carbonate. Aluminium compounds are excellent binders, but are not recommended over long periods, given that they may induce aluminium intoxication in CKD patients.
Treatment of vitamin D deficiency
• Cholecalciferol (D3): Belenguer® or Kern®vitamin D3 (cholecalciferol, 800IU = 12 drops, 50 000IU = 25ml).
• Calcifediol (25-OH-vitamin D): Hidroferol® 0.266mg (calcifediol 16 000 IU).
The purpose of fortnightly or monthly calcifediol administration in vials is to normalise calcidiol levels (25-OH >20-30ng/ml) independently or not of a decrease in PTH. It should be managed with the utmost care and it is necessary to measure calcium and phosphorus in its control, since in patients with advanced CKD, it may increase them.
Treatment of secondary hyperparathyroidism
• Calcitriol and vitamin D analogues: renal hydroxylation is not required to obtain the active form. These include Rocaltrol® (calcitriol; 1,25-(OH)2-D3) and Etalpha® (alfacalcidol; 1α-(OH)-D3). Alfacalcidol requires activation in the liver.
• Selective vitamin D receptor activators:Zemplar® (paricalcitol): there is a lower likelihood of hypercalcaemia and hyperphosphataemia, and it seems to induce fewer vascular calcifications.
• Calcimimetics:Mimpara® (cinacalcet): indicated in the treatment of hyperparathyroidism in dialysis and in primary hyperparathyroidism.
Detection and treatment of acidosis
A situation of metabolic acidosis not compensated with venous bicarbonate <15mmol/l will require intravenous treatment in the hospital. Mild metabolic acidosis (bicarbonate between 15 and 20mmol/l) may require the administration of oral bicarbonate.
Other attitudes
Preparation for renal replacement therapy and start time
The optimal initiation of renal replacement therapy (RRT) is that which is planned. The lack of planning unnecessarily increases the use of catheters in HD, causing higher morbidity, infections and increased hospitalisation.
A referral of the patient to the nephrologist at the appropriate time means that the patient will receive the best information on the possible RRT techniques: PD, HD and home HD, as well as the possibility of an early or living donor renal transplantation, if this were available. This adequate referral decreases complications, particularly infectious and cardiovascular complications and has a significant impact on survival.
RRT is considered when the GFR is <15ml/min/1.73m2 or earlier if there are signs or symptoms of uraemia or a difficulty in controlling hydration (carried out frequently in diabetic patients), HBP that is difficult to control or a deterioration of nutritional status.
In general, dialysis is introduced whenever the GFR is between 8 and 10ml/min/1.73m2 and it is essential with a GFR <6ml/min/1.73m2, even in the absence of symptoms of uraemia. In high-risk patients, we insist that the early introduction of dialysis should be considered and it should be personalised.
It is necessary to bear in mind that the patient may be suitably studied and prepared for a potential living donor renal transplantation (if this is possible) before dialysis is initiated. Likewise, they can be studied and placed on the waiting list for a deceased donor renal transplantation, where possible, before starting dialysis. This is what we call early renal transplantation.
Follow-up
Patients with stages 4-5 CKD should be monitored preferably by the nephrologist, in close collaboration with the Primary Care doctor and the nursing staff.
The frequency of visits must be established every three months in stage 4 CKD and every month in pre-dialysis stage 5 CKD. This frequency may be modified according to the doctor’s opinion.
In each visit, it is advisable to provide detailed information about the laboratory test, changes to treatment, its justification and, where appropriate, a prognostic assessment.
Roles of the Primary Care doctor in addressing and following up chronic kidney disease determined by the stage of disease
• Follow-up of elderly patients, with a stable GFR, who due to age, quality of life or other reasons, do not receive RRT, ESA and/or medication for secondary hyperparathyroidism.
• Control of cardiovascular risk factors.
• Monitoring of CKD progression factors.
• Monitoring of nephrotoxicity in order to avoid iatrogeny in any process.
• Special attention must be paid to:
- Avoiding NSAID use, whenever possible.
- Avoiding hyperkalaemia associated with the use of drugs.
- Avoiding/adjusting the use of oral anti-diabetics in accordance with the eGFR.
- Avoiding, insofar as possible, use of iodine contrasts and adjusting all drugs to the patient’s eGFR.
• Participation in therapeutic compliance and referral to Nephrology in the case of acute deterioration of renal function or complications.
• Vaccination: hepatitis B virus, pneumococcus, flu, etc.
• Collaboration in palliative activities.
Keys for managing haemodialysis/peritoneal dialysis patients in Primary Care
• Facilitation of the disease adaptation process in accordance with age, the family situation, educational and work conditions, the form of occurrence and development of the disease, trust in the healthcare system, etc.
• Knowledge by the GP of the different options and implementation of the latter (frequency, location where it is performed, potential complications according to the alternative chosen).
• Good relationship and communication channel with the reference Nephrology Service.
Keys for managing kidney transplant patients
The same applies as in the previous case but with a very intense interaction with the nephrologist, for special requirements related to immunosuppression, pharmacological interactions and vaccinations.
Keys for follow-up of terminal uraemia at home. Palliative treatment
The objective of home management of terminal uraemia is to facilitate the wellbeing of uraemic patients who cannot undergo dialysis, minimising the physical, family and healthcare impact of their condition and optimising the resources of our National Health System. This requires close coordination between Nephrology and Primary Care. In health areas where there are home support teams, whether they are dependent on Primary Care or Specialist Care, their inclusion in the therapeutic team could be very useful.
The individualisation of the decision with the agreement of the patient, family and professionals is recommended. It is useful for the decision to be made at an early stage, since it allows follow-up to be organised before a very significant deterioration has occurred in the patient.
Acknowledgements
We would like to thank Esteve and Abbvie laboratories for their logistical support in the project.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Chronic kidney disease risk factors
Table 2. Equations to use in methods for measuring creatinine without traceability to IDMS (non-standardised)
Table 3. Equations to use in methods for measuring creatinine with traceability to IDMS (standardised)
Table 4. Prognosis of chronic kidney disease by estimated glomerular filtration rate and albuminuria (6)
Table 5. Predictors of chronic kidney disease progression
Table 6. Frequency of monitoring visits (number of annual visits)
Table 7. Objectives by specialty in chronic kidney disease patient follow-up
Figure 1. Algorithm of referral to Nephrology.