Infection with the coronavirus SARS-CoV-2, causing COVID-19, originated in Wuhan, China, in December 2019.1 This infection quickly propagated around the world, affecting numerous countries, and was declared a pandemic by the World Health Organization on 11 March 2020.
Subgroups at risk of developing severe forms of the disease have been identified, including kidney transplant patients, who have a greater incidence of infection and admission, as well as a greater need for admission in intensive care units.2 Immunosuppressant treatment is one of the reasons for this population's vulnerability.
The management of immunosuppressant treatment in a context of SARS-CoV-2 infection is unclear.3,4 In addition, the safety and interaction profile of certain drugs used to treat the infection limits their use. Within this treatment group, intravenous non-specific human immunoglobulins (IV Ig) may have a good profile, making them an interesting alternative treatment.5
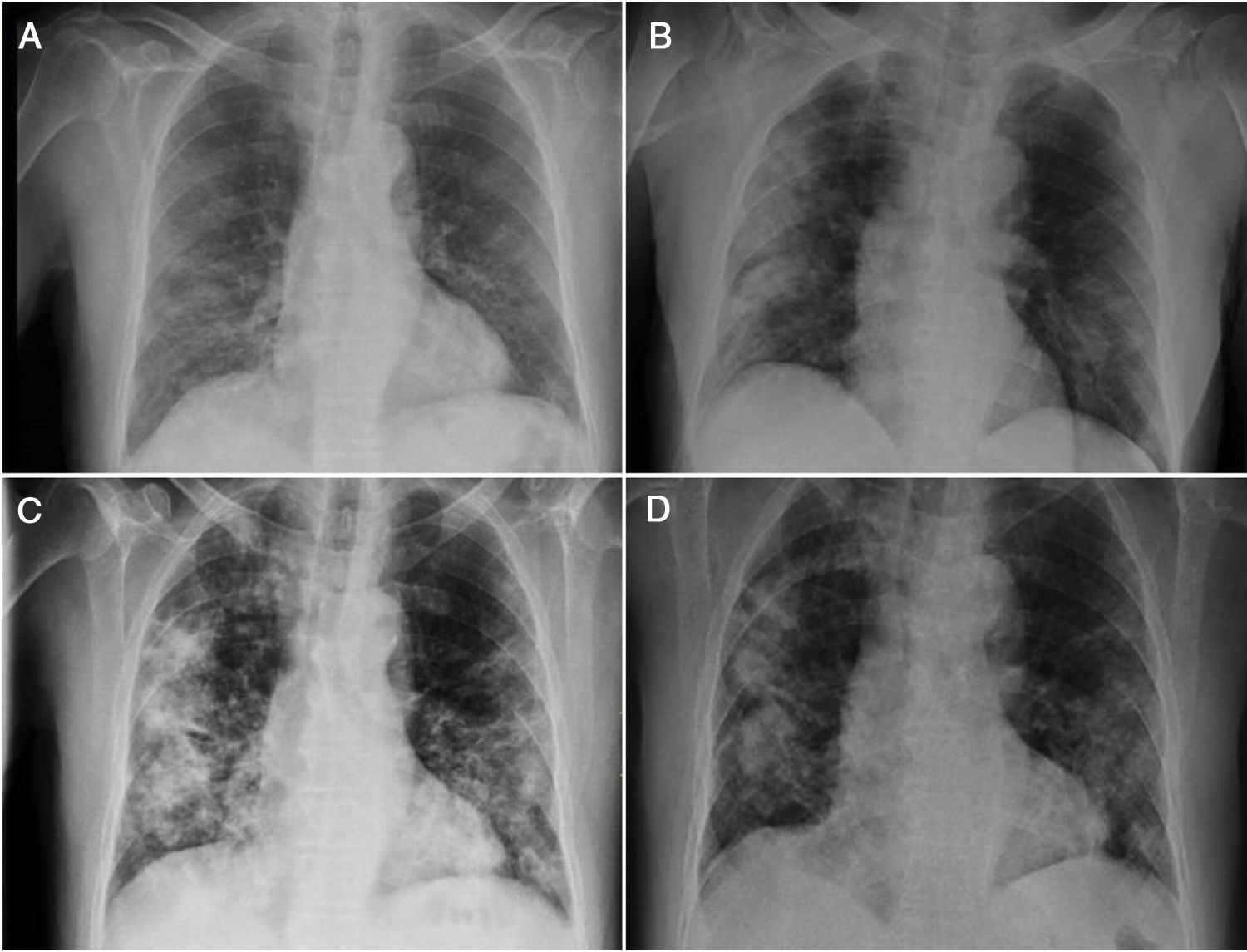
We report the case of a 72-year-old man with hypertension and dyslipidaemia who underwent kidney transplantation in 2012. He was on immunosuppressant treatment with tacrolimus and everolimus and had a baseline creatinine level of 1.43mg/dl. The patient visited the emergency department where he reported experiencing general malaise and headache for the previous three days. He had normal blood pressure levels, an axillary temperature of 37°C and an oxygen saturation (O2 sat) of 92% breathing room air. A chest X-ray showed bilateral pulmonary interstitial infiltrates (Fig. 1A). Laboratory testing revealed kidney failure (creatinine 2.52mg/dl and urea 77mg/dl) and higher levels of tacrolimus (15.1ng/ml) compared to his most recent visit. Polymerase chain reaction (PCR) testing of the patient's nasopharyngeal discharge was positive for SARS-CoV-2. A decision was made to admit him and start treatment with hydroxychloroquine, azithromycin and lopinavir/ritonavir along with ceftizadime and glucocorticoids (Table 1). The immunosuppressant treatment the patient was on was suspended.
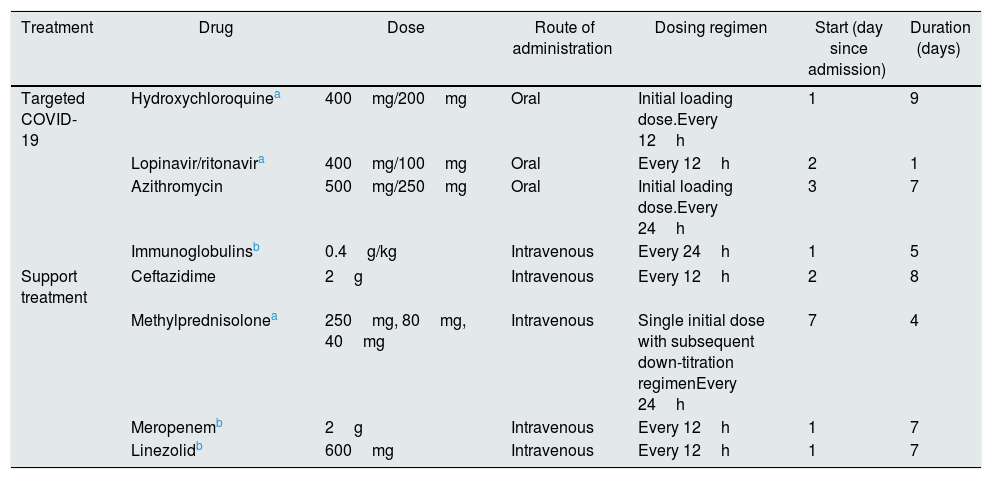
Summary table of the treatments administered during the patient's first and second admissions.
| Treatment | Drug | Dose | Route of administration | Dosing regimen | Start (day since admission) | Duration (days) |
|---|---|---|---|---|---|---|
| Targeted COVID-19 | Hydroxychloroquinea | 400mg/200mg | Oral | Initial loading dose.Every 12h | 1 | 9 |
| Lopinavir/ritonavira | 400mg/100mg | Oral | Every 12h | 2 | 1 | |
| Azithromycin | 500mg/250mg | Oral | Initial loading dose.Every 24h | 3 | 7 | |
| Immunoglobulinsb | 0.4g/kg | Intravenous | Every 24h | 1 | 5 | |
| Support treatment | Ceftazidime | 2g | Intravenous | Every 12h | 2 | 8 |
| Methylprednisolonea | 250mg, 80mg, 40mg | Intravenous | Single initial dose with subsequent down-titration regimenEvery 24h | 7 | 4 | |
| Meropenemb | 2g | Intravenous | Every 12h | 1 | 7 | |
| Linezolidb | 600mg | Intravenous | Every 12h | 1 | 7 | |
Following five days of admission, during which the patient responded favourably (laboratory testing showed that his inflammatory parameters decreased and his kidney function returned to normal), he presented an increase in tacrolimus levels (29.31ng/ml), which returned to normal after four days. Another X-ray was taken and ruled out progression (Fig. 1B). A decision was made to discharge him from hospital and restart his prior immunosuppressant treatment at his usual doses.
Seven days later, the patient returned to the emergency department and reported experiencing fever in recent days, with gradually increasing dyspnoea accompanied by micturition syndrome. A new chest X-ray showed worsening compared to the patient's prior chest X-ray (Fig. 1C). Laboratory testing showed leukocytosis (16,400/μl) with lymphopenia (600/μl) and elevated acute-phase reactants (C-reactive protein [CRP] 7.6mg/dl, D-dimer 886μg/l and procalcitonin 3.88ng/ml). Treatment was started with IV Ig plus linezolid and meropenem. The patient was admitted (Table 1) and his prior immunosuppressant treatment was resuspended.
Five days of treatment with IV Ig were completed. The patient did not present any adverse reactions and did show marked clinical improvement; his dyspnoea disappeared and he showed improvement on X-ray (Fig. 1D). This allowed him to be discharged from hospital early, on day six.
The case reported reflects the complexity of treating SARS-CoV-2 infection in a kidney transplant patient, as the patient's treatment was found to be limited by drug interactions. In this case, the interaction between tacrolimus and lopinavir/ritonavir was assumed to be the main reason for the patient's elevated tacrolimus levels, though the concomitant decline in kidney function may have contributed.6 Management of immunosuppressant treatment in transplant patients with COVID-19 is debated. Although some published reports are available, the repercussions of maintaining usual immunosuppressant doses in the context of this infection are unknown.3,4,7
Moreover, SARS-CoV-2 infection still lacks effective treatment. The limited benefits of lopinavir/ritonavir seen in recent studies cast doubt on their usefulness, especially in patients who are more susceptible to their toxic effects.8
Another group of drugs that have been gaining reputation in the control of severe forms of infection are biologic drugs. However, their use is restricted in previously immunosuppressed patients. In this regard, treatment with IV Ig is gaining importance and represents an appealing option due to their safety profile and their potential efficacy achieved by means of modulation of inflammatory response. While evidence supporting their use is limited, recently published cases point to a possible benefit in severe forms of COVID-19.9 Their use has also been associated with a good clinical outcome in kidney transplant patients.10 The case reported constitutes the first in Spain supporting the use of IV Ig in kidney transplant patients with severe forms of COVID-19.
Please cite this article as: Sánchez Cadena AD, Negreira Caamaño M, Pérez Serrano R, Porras Leal ML. Inmunoglobulinas por vía intravenosa: una alternativa terapéutica a tener en cuenta en el paciente trasplantado renal con COVID-19. Nefrologia. 2021;41:220–222.