The measure of intraperitoneal pressure in peritoneal dialysis is easy and provides clear therapeutic benefits. However it is measured only rarely in adult peritoneal dialysis units. This review aims to disseminate the usefulness of measuring intraperitoneal pressure. This measurement is performed in supine before initiating the drain of a manual exchange with “Y” system, by raising the drain bag and measuring from the mid-axillary line the height of the liquid column that rises from the patient. With typical values of 10–16cmH2O, intraperitoneal pressure should never exceed 18cmH2O. With basal values that depend on body mass index, it increases 1–3cmH2O/L of intraperitoneal volume, and varies with posture and physical activity. Its increase causes discomfort, sleep and breathing disturbances, and has been linked to the occurrence of leaks, hernias, hydrothorax, gastro-esophageal reflux and enteric peritonitis. Less known and valued is its ability to decrease the effectiveness of dialysis significantly counteracting ultrafiltration and decreasing solute clearance to a smaller degree. Because of its easy measurement and potential utility, should be monitored in case of ultrafiltration failure to rule out its eventual contribution in some patients. Although not yet mentioned in the clinical practice guidelines for PD, its clear benefits justify its inclusion among the periodic measurements to consider for prescribing and monitoring peritoneal dialysis.
La medida de la presión intraperitoneal en diálisis peritoneal es muy sencilla y aporta claros beneficios terapéuticos. Sin embargo, su monitorización todavía no se ha generalizado en las unidades de diálisis peritoneal de adultos. Esta revisión pretende divulgar su conocimiento y la utilidad de su medida. Se realiza en decúbito antes de iniciar el drenaje de un intercambio manual con bolsa en Y, elevando la bolsa de drenaje y midiendo la altura que alcanza la columna de líquido desde la línea medio-axilar. Los valores habituales son 10 a 16 cmH2O y nunca debe superar los 18 cmH2O. Aumenta de 1 a 3 cmH2O por litro de volumen intraperitoneal sobre valores basales que dependen del índice de masa corporal y varía con la postura y la actividad física. Su aumento provoca malestar, alteraciones del sueño y de la respiración, y se ha relacionado con la aparición de fugas de líquido, hernias, hidrotórax, reflujo gastroesofágico y peritonitis por gérmenes intestinales. Menos conocida y valorada es su capacidad para disminuir la eficacia de la diálisis contrarrestando, sobre todo, la ultrafiltración y, en menor grado, el aclaramiento de solutos. Por su facilidad de medida y potencial utilidad, debería ser uno de los factores que investigar en los fallos de ultrafiltración, pues su elevación podría contribuir a ellos en algunos pacientes. Aunque todavía no se menciona en las guías de actuación en diálisis peritoneal, sus claros beneficios justifican su inclusión entre las mediciones periódicas que considerar para la prescripción y seguimiento de la diálisis peritoneal.
In pediatric peritoneal dialysis (PD) infusion volume is calculated using objective criteria taking into consideration the size or intraperitoneal pressure (IPP). In adults the guidelines do not provide specific recommendation and the prescription of infusion volume is usually based on size of the patient, weight and body surface, without taking into account the IPP. In any case, this volume infused increases the IPP which may cause discomfort, fullness, sleep disturbances, hemodynamic and respiratory alterations and it is believed that contribute to certain mechanical complications (leakage, hernia, etc.).1,2 Is less known the effect of IPP on the efficacy of dialysis, mainly through a reduction of ultrafiltration (UF),3–5 and this is the aspect that we like to discuss here in more detail. Since we are convinced of the advantages of its simple determination, we want to disseminate its knowledge and promote its routine use for a rational prescription of infusion volume in PD and in the management of UF disorders.
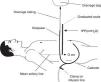
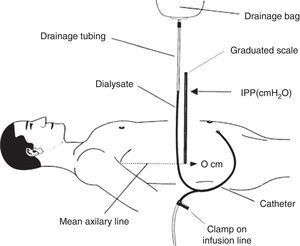
How is intraperitoneal pressure measured in peritoneal dialysis?The IPP in PD is measured by noninvasive methods. The simplest and safest method was published by Durand in 19926,7 (Fig. 1); it measures the IPP in the filled abdomen before drainage. The patient should be supine on a horizontal position, relaxed and with the head supported so abdominal wall is relaxed to avoid pressure on the abdomen. A “Y” system PD bag is attached and the drainage bag is held in a raised support; a graduated ruler is placed next to the line going from the patient up to the bag and aligning level 0 with the mid axillary line. Then, the catheter connection is opened (in systems with wheel, stay-safe® type, it is aligned to the drainage position) and the column of liquid rises to a level where it stabilizes with a respiratory oscillation of 1–2cmH2O which guarantees a correct measurement. The IPP will be measured as the midpoint of that oscillation, and is expressed in cm of H2O.1,7 Once the measurement has been obtained, the abdomen is drained and the volume is recorded. To measure IPP sitting or standing, the point “0” is considered in the mid-axillary line at the midpoint between the xiphoid and pubic symphysis8,9 or in the anterosuperior iliac spine.10 In order to measure the IPP in moments different of PD exchange, a conventional venous pressure measurement system must be connected to the catheter either directly or via a 3-way valve.
Scheme of intraperitoneal pressure measurement (IPP) using a ruler and the drain line of a PD bag with Y system.
Source: Mathieu et al.,7 with permission.
We considered that in routine clinical practice the IPP should be measured as part of the initial assessment of the clearance and peritoneal function of each patient, and when the volume of infusion is modified. IPP measurement should be associated with the peritoneal equilibration test to correlate it with UF data and solute clearance. It should be measured if there are problems with UF.
Normal values of intraperitoneal pressure in peritoneal dialysisIn stable adult PD patients with 2L of dialysate a IPP of 10–16cmH2O on the mid-axillary line is considered aceptable.1,6,11–15 It should be kept below 18cmH2O because higher values are associated with symptoms.4,12,16 Some authors use the umbilicus or other points as the point zero. Others express the IPP in mmHg (1.0mmHg=13cmH2O).
Low IPP values may allow to increase the intraperitoneal volume (IPV) with the consequent increase in clearance capacity17; however, the efficacy of PD is affected by IPP even with values in the low segment of the normal range.18 Patients with Kt/V and UF in the limit, and with low tolerance to PD may do better with lower IPP values.19,20
Normal range of IPP in children is the same as in adults, from 5 to 15cmH2O and the maximum of 18cmH2O, although personal tolerance has to taken into consideration with a significant individual variation that is related to a more variable body mass index (BMI)
Factors influencing intraperitoneal pressureWith an empty peritoneum the IPP values depend on body size and BMI23 and increase with IPV, body position, and physical activity (Figs. 2–4), and is less conditioned by other factors.
- (1)
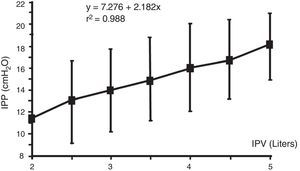
The IPV which is sum of the previous residual volume, volume infused and UF volume, is the main factor that affects IPP.6,8,14,22,24,25 It rises from 1 to 3cmH2O (average of 2.2cmH2O) per liter above the void IPP.16 This increase is very regular in each patients, so with a IPP measurement with a known IPV, further change IPP for additional volumes can be estimated in the same patient.15 This regular increase of IPP with IPV in the same patient makes a great contrast with the great interpatient IPP variation: in different patients with a given IPV the IPP may vary more than 10cmH2O, and in different patients with the same IPP the IPV may vary more than 6L (Figs. 2 and 3). So, in a patient done IPP cannot be deduced until the patient has had its own measurements.12,24
- (2)
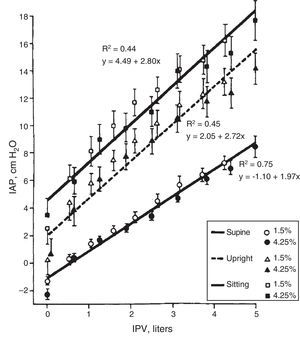
Body Position. The IPP is low if the patient is lying down and it increases 2–4cmH2O in standing position (this increase in IPP is magnified by IPV). The IPP is even higher when seated producing an additional increase of 1.5–2cmH2O (Fig. 3).8–10 Automated PD (APD) is performed during the night hours with the lowest IPP.
- (3)
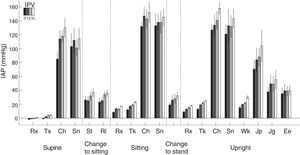
Physical activity: This is the factor that causes a greatest increases in IPP (Fig. 4), since it may multiply the basal values by 15 in proportion to the intensity of the exercise. The relationship of IPP values and body positions is maintained; sitting>standing>lying down. Complications related to excessive IPP such as hernias, fluid leakage, etc., are probably more related to these large and incidental increases in IPP, precipitated by cough and isometric exercise, rather than sustained increase in IPP in moderate basal levels. This also marks differences between continuous ambulatory peritoneal dialysis (CAPD) and APD especially with dry day.
- (4)
BMI: In both children4,21 and adults,1,14,15,26 there is a marked correlation between IPP and BMI (weight/height2). Thus more obesity is associated with higher IPP. This correlation is so strong that the interindividual variation in BMI would largely explain the variability of IPP in among the children21 and adults (unpublished own data). Some authors do not observe this correlation.11,20
- (5)
Other factors: in adults a greater body surface area has been associated with lower IPP.27 Apparently, gender does not make a difference in IPP.14,15,26 Perhaps because of a reflex contracture, the IPP is relatively increased during the first days following the implantation of the PD catheter18 and during the first hour of exchanges with volumes greater than 2L.28,29 Small differences have also been detected with the use of different PD solutions.19,20
Relationship between intraperitoneal pressure (IPP) and intraperitoneal volume (IPV). Note that different patients achieve the same IPP with highly variable intraperitoneal volumes, 2–5L.
Source: Durand et al.,12 with permission.
Correlationship between intraperitoneal pressure (IPP) and intraperitoneal volume (IPV) in different body positions, with glucose solutions 1.5 and 4.25% (mean±SEM).
Source: Twardowski et al.,8 with permission.
Intraperitoneal pressure (IPP) during natural activities with different intraperitoneal volumes (IPV) (0, 1, 2 and 3L) in 6 patients. Note the large difference of basal values at rest, on the left, with all the others.
Rx: rest; Tx: talking; Ch: coughing; Sn: stretching; St: sitting up; Rl: rotating; Tk: talking: Wk: walking; Jp: jumping; Jg: jogging; Ee: on a static bicycle.
Source: Twardowski et al.,25 with permission.
In PD patients, elevated IPP increases morbidity,29 mortality and the need of hemodialysis due to failure of PD technique.26 A progressive increase in IPP at rest may cause a sudden decrease in respiratory indexes8,12,16,30 and in this situation patients sometimes present a symptomatology12 of malaise,27 abdominal or back pain, a superficial respiration and sleep disorders, including sleep apnea.12,27,30 These symptoms are more intense in decubitus and becomes more patent in patients on APD. Apart from its impact on the quality of life, these symptoms may induce a lack of compliance with the consequent reduction in the dose of dialysis.16,22 There have been major complications and even deaths29 associated with overfilling of the peritoneal cavity due to catheter or cycler malfunction, patient errors, etc., These serious adverse events are probably related to the increase in IPP and the important dysfunctions derived from the hemodynamic effects of elevated IPP seen in surgical patients or with multiorgan failure.23
There are mechanical complications of PD that are related to an increase in IPP as hydrothorax, hernia, leakage, peritoneal-vaginal hydrocele, genital edema, gastroesophageal reflux, hemorrhoids, etc.1,2 It has been shown that hernias are more frequent in PD than in hemodialysis13 and more in CAPD than in APD,31 especially with basal IPPs above 20cmH2O,1 although not always hernias are associate with higher basal IPPs.26 These complications appear to be more influenced by the technique of catheter implantation, sudden elevation of IPP due to cough or other physical exercises (Fig. 4), small body size, individual predisposition1,32 or other characteristics.2,16,33 Higher IPPs have also been associated with a higher incidence of peritonitis caused by germs of intestinal origin15 and also with an increase in the local production of endothelin, which is related to long-term deterioration of the peritoneum.2
Increasing filling volumes to augment PD clearance may cause complications if the IPP reaches values above 18cmH2O.24,27,30 Some patients achieve this high IPP with 2L and others with more than 5L (Fig. 2)12,24 and direct measurement of IPP is the only way to know it.
Effects on the efficacy of peritoneal dialysisThe IPP modulates the efficacy of PD significantly, mainly by affecting UF volume, but also by modifying the clearance of solutes.
UltrafiltrationThe UF capacity of PD is lower than that of hemodialysis, which explains a higher incidence of overhydration PD patients, resulting in increased morbidity and mortality and is an important cause of change to hemodialysis.34 The capacity to ultrafiltrate is a better index of the treatment outcome than the solute clearance.35 Therefore, the clinician must know how to optimize any of the factor influencing UF in PD. Although the general believe is that in PD the UF is driven only by osmotic gradient, there are other forces that affect UF such as IPP and also Starling's forces (hydrostatic and intra- and pericapillary oncotic pressures) which govern the transcapillary transport of fluids.
- (1)
IPP: since 1981 it is known that the decrease in the net UF that occurs in the long exchanges of PD is due to the reabsorption of the peritoneal fluid.36 In 1983, this resorption was shown to be proportional to the increase in IPP37 and Durand in 1992 alerted that, in adults in PD, the increase in IPP within the normal range from 8 to 18cmH2O produced a proportionally reduction of the UF volume obtained with 3.86% glucose solution.6 Fischbach observed in children with low IPVs (1000mL/m2) and IPP below 8cmH2O, that these low IPP pressures do not reduce the large UF of 3.86% glucose but it may decrease the modest UF of 1.36% glucose.18 Other authors also observed that from very low values the elevation of IPP counteracts the UF, although this effect can still be overcome by increasing the osmotic gradient.38 Since then it has been confirmed that the increase of IPP in PD significantly neutralizes the UF produced by the osmotic gradient.3,4,6,18,20,22,38–40 However, with the use of icodextrin, which causes slower UF, the effect is not apparent in the first 4h of permanence.20 Although the effect of IPP on UF has never been discussed, it has generally been ignored and its intensity41,42 and significance in clinical practice has been questioned; only indirect evidence is found in clinical studies evaluating the performance of the UF using different volumes,28,38,43–46 changes in body position47 or in special situations.48
It is not easy to explain the interrelation of UF with IPV and IPP. The osmotic gradient is maintained for a longer period of time if the volume of infusion is increased,43 therefore the UF increases but only until the increase in IPV produces an elevation of IPP.38,43 The osmotic effect only prevails if it is strong. In general, using 1.36% glucose, we observe a decrease in the net UF44 and with 2.27% glucose there is a decrease in the relative UF (measured as percentage of the IPV),28,45 and it is necessary to use 3.86% glucose to increase the net UF volume with higher IPVs.38 However Paniagua et al.,43 in 30 unscreened patients, documents an increase of net UF with elevated IPV using 1.36% glucose, despite an increase in IPP.
Criticism of some experts in relation to the effect of IPP on peritoneal transport is based on the fact that IPP it does not appear to affect significantly the transcapillary transport of fluids in the peritoneum.41,42 The reduction of UF caused by high IPP seem to be explained by reabsorption of peritoneal fluid which are difficult to evaluate. There appear to be three mechanisms by which IPP counteracts osmotic UF in PD5,41,49:
- (A)
Peritoneal fluid reabsorption by increase in the rate of lymphatic absorption. This contributes by a 15–25% of the total resorption.50 The both lymphatic absorption and lymphatic circulation depends directly on hydrostatic pressure,9,11 therefore the higher the IPP, the greater the lymphatic flow and the lower the net UF.4,19,27,41
- (B)
Tissue reabsorption: the diffusion out to the surrounding tissues, such as muscles of the abdominal wall,5,37,39,50 is the most important component of increased resorption induced by high IPP.
- (C)
Reduction of transcapillary filtration, which appear to have less significance, is the result of two effects of Starling equilibrium:
- (a)
Elevation of IPP is transmitted to the peritoneal interstitium which decreases the gradient of transcapillary hydrostatic pressure, which reduces the escape of water from the capillaries out to the interstitium.40 However Rippe et al.41 rationalizes that this effect of high IPP is neutralized by itself increasing not only the interstitial pressure but also the venous pressure, which will help to maintain the transcapillary pressure gradient.
- (b)
In any case, the inflow of fluid into the peritoneal interstitium induced by IPP causes edema, with a corresponding decrease in interstitial oncotic pressure, which increases the colloid-osmotic transcapillary gradient, the other Starling force that increase the capillary recruitment of fluid.41
- (a)
This effect of IPP diminishing UF in PD is not yet considered relevant in clinical practice and consequently is rarely measured and, except in some cases51,52 is not mentioned in articles or handbooks on causes and treatment of UF failure. In fact IPP is not mentioned in the different guidelines for PD.
- (2)
Starling forces (hydrostatic and oncotic intra- and pericapillary pressures) governing transcapillary fluid transport. (A) Intracapillary hydrostatic pressure: increases with fluid overload and favors UF together with osmotic gradient.46,48 The intracapillary hydrostatic pressure decreases in situation of volume depletion. (B) Intracapillary oncotic pressure, which is low in patients with hypoalbuminemia, not uncommon in PD patients due to nutritional deficit, peritoneal losses of proteins and hemodilution associated to overhydration. Low intracapillary oncotic pressure causes a reduction of the transcapillary oncotic gradient resulting in a decreases in the reabsorption of fluids which favors UF.41,42
Thus, the net UF volume in PD is the net result of 4 forces: (1) the osmotic pressure, the most powerful and the only one that we can deliberately control and therefore the only one that is taken into consideration. The other 3 additional forces are not usually considered as related to UF: (2) the IPP that depends on IPV, UF volume, body position, BMI, physical activity, etc.; (3) capillary hydrostatic pressure that varies with the degree of water overload and (4) capillary oncotic pressure that is proportional to albumin concentration, which in turn is related to the degree of volume overload. These pressures interact in every moment in unknown proportions, so it is understandable that the clinical importance of IPP, transcapillary hydrostatic pressure and oncotic pressure remain unnoticed for clinicians. The role of each of these pressures will be better interpreted if, in addition to volume and concentration of PD fluid infused, we measured IPP and consider the role of transcapillary hydrostatic pressure by assessing the degree of hydration with methods such as vectorial bioimpedance. With these data, in presence of an UF failure we could analyze all its components and optimize the therapeutic strategies including modifications of PD solution, dwell time, changes in IPV, body position (DPCADPA), physical activity and, at the long term, reducing obesity (BMI) and correcting hypoalbuminemia.
UF failure is attributed to peritoneal membrane defect when mechanical and reversible causes are ruled out and if the UF volume achieved with the peritoneal equilibration test with 3.86% glucose is less than 400mL.52,53 Then, if the patient is not classified as high (membrane failure type I) or low transporter (type II) and the sieving of sodium excludes aquaporin abnormality, by exclusion, the patient has a ‘high lymphatic transport’ (type III52,54,55 or type IV34). This abnormality could be confirmed by testing the clearance of macromolecules, a test that is impracticable in standard clinic.56 And that the test of clearance of macromolecules measures actually is the sum of lymphatic reabsorption and tissue diffusion, the main forces by which the increase of IPP counteracts the net UF, even without peritoneal membrane defect. If IPP is measured associated to peritoneal equilibration test it would be possible to know if IPP values has a role in these cases of UF failure and if a more specific approach can be applied.
Clinical studies that consider IPP to treat UF failure are still scarce.5,34,46,48 The “adapted APD” of Fischbach's35 is an attempt to optimize UF and clearance in PD by combining short low volume exchanges with longer and higher volume exchanges. Short low volume exchanges improves UF, not only because in short time osmotic gradient is maintained, but because, as the low volume do not increase IPP. Rippe et al., doubts about the effectiveness of this strategy.42 All these experiences indicate that an understanding of how IPP affects UF could improve the management of UF filure and avoid transference to hemodialysis in some cases.
Therefore, in PD the IPP, even in normal values, significantly reduces the UF and could be in some patients a relevant cause of UF failure that could be corrected using adequate measures. The present review aims to disclose the advantages of measuring IPP for management and monitoring patients in PD, especially in situations of UF failure and water overload.
Clearance of solutesA number of manuscripts have evaluated the effect of IPP on the PD clearance of low molecular weight molecules (urea, creatinine, sodium, potassium, and phosphate). An increase in IPP, not secondary to an elevation of IPV (by external compression), produces a reduction of solute clearance.9,40,47 If the increase in IPP is produced by an elevation of IPV aiming to increase clearance, the reduction of clearance by high IPP is compensated by the increase in clearance induced by volume; net clearance my be increased mainly if the fluid contains a high glucose concentration17,28,38,43 although the clearance relative to volume decreases.17,28,30,38,57
ConclusionsThe measurement of IPP is simple and harmless and helps to optimize prescription and monitoring of PD and assessment of adequate IPV that prevents mechanical complications and reduction of UF. Elevation of IPP is a cause of UF failure easily assessed or ruled out.
Key conceptsIn PD the peritoneal fluid increases IPP which may cause discomfort and decreases the efficacy of PD, especially UF.
The measurement of IPP in PD is easily performed in decubitus position before the drainage of a manual exchange with a Y-bag, raising the drainage bag and measuring the height of the column of fluid over the mid-axillary line. Usual figures are 10–16cmH2O and, values above 18cmH2O causes discomfort.
The IPP increases about 2–2.5cmH2O per liter of IPV and this is very constant between patients. But since the basal IPP values are variable from one patient to another, we must measure the IPP to be sure that the maximum level of 18cmH2O is not to exceeded.
The IPP is proportional to the BMI, is lower in decubitus position, increases standing, and increases even more if seated and, above all, with physical activity.
The increase in IPP due to IVP causes discomfort, sleep disturbances and breathing difficulties and has been associated with hernias, fluid leakage, hydrothorax and gastroesophageal reflux.
High IPP increases reabsorption of the peritoneal fluid and significantly decreases UF, even with IPP values within the normal range. In high transporters or anuric patients, an increase in IPP may cause UF failure that can be improved by reducing IPV for as long as sufficient clearances can be maintained.
Thus, IPP should be considered one reversible cause of UF failure since the beginning of the diagnosis process.
Conflict of interestsNone of the authors declares a conflict of interest in relation to the content of this work.
Please cite this article as: Pérez Díaz V, Sanz Ballesteros S, Hernández García E, Descalzo Casado E, Herguedas Callejo I, Ferrer Perales C. La presión intraperitoneal en diálisis peritoneal. Nefrologia. 2017;37:579–586.