Normal saline has traditionally been the resuscitation fluid of choice in the perioperative period of kidney transplantation over balanced potassium solutions. However, the problems arising from hyperchloraemia triggered by the infusion of normal saline have led to studies being conducted that compare this solution with balanced solutions. From this narrative review it can be concluded that the use of balanced crystalloids containing potassium in the perioperative period of kidney transplantation can be considered safe. These solutions do not affect serum potassium levels any more than normal saline, whilst maintaining a better acid–base balance in these patients.
El suero salino normal (SSN) ha sido clásicamente el fluido de resucitación elegido en el periodo perioperatorio del trasplante renal frente a aquellas soluciones balanceadas con potasio. Sin embargo, los problemas derivados de la hipercloremia desencadenada por la infusión de SSN han llevado a la realización de estudios que comparaban esta solución con los fluidos equilibrados. Mediante la presente revisión narrativa se deduce que el uso de cristaloides balanceados con contenido de potasio en su formulación, en el perioperatorio de trasplante renal, puede considerarse seguro. Estas soluciones no provocan una alteración del potasio sérico mayor que la provocada por el SSN y mantienen mejor el equilibrio ácido-base en estos enfermos.
Fluid and electrolyte replacement during the post-transplant period aims to maintain an adequate intravascular volume to ensure renal perfusion so immediate graft function is optimized. To achieve this goal, an adequate understanding and management of fluid therapy is essential; a major surgery is commonly associated to renal insufficiency and electrolytic disorders such as hyperkalemia that should be prevented and the function of the graft needs to be warranted.1
Delayed graft function is a term used to describe acute renal failure after transplantation and may be defined by the need for dialysis during the first postoperative week. Delayed graft function is important predictor of the subsequent clinical course of the graft.1,2 There are several factors that are related to a delayed graft function: age of donor, quality of the tissues, cold storage, reperfusion injury, prerenal causes, immunosuppressive drugs, etc.3,4 Likewise, the presence of hyperkalemia may contribute to graft dysfunction.5 Classically, normal saline (NS) has been chosen during the perioperative period of renal transplantation. This choice has been based on the belief that the use of potassium containing replacement fluids could produce hyperkalemia.6
However, there are views that attribute to NS an increase of serum chlorine that predispose to the development of metabolic acidosis and the generation of hyperkalemia through a transcellular movement of ions. This concept has been that basis for the elaboration of several studies during the last decade comparing the use of NS and balanced crystalloid solutions (including potassium in their formulation) during the perioperative period of renal transplantation.7–11
The present short reviews is brief pathophysiological assessment of this concept as well as a description of the publications in the current medical literature.
Type of fluidsIntravenous fluids are separated into 2 types: crystalloids and colloids. Crystalloids are made of sterile water and electrolytes and sometimes contain glucose as a source of calories. Colloids are solutions containing high molecular weight particles that increase oncotic pressure and are added to a crystalloid. This group includes albumin, gelatins, dextrans and starches (derived from corn and potato).12
The increase in oncotic pressure increases intravascular fluid retention capacity as compared to crystalloids. This theory is based on the theoretical premise that larger particles are trapped in the intravascular space by an intact endothelial barrier for longer period of time.13
However, it is necessary to consider that a colloid only behaves as a colloid (that is, increasing oncotic pressure) when the glycocalyx is intact.14 In fact, in the perioperative period (in situations such as preoperative fasting) bleeding and insensible losses can reduce the extracellular volume and activate the inflammatory cascade, with consequent damage of the glycocalyx, which increases capillary permeability and looses of intravascular fluids.15,16 This fact explains why large clinical trials observed that the advantage in volume expansion is generally only about 30–40% in favor of colloids, far from theoretical potency in situations of intact glycocalyx.17–19
Furthermore, the use of colloids increases the cost, have limited availability (the case of albumin, which is a blood product) and are associated with clotting disorders that may cause persistent renal damage, mainly observed with the use of hydroxy-ethyl starches.20–23
These details may have led clinicians to choose crystalloids as the first option in the postoperative period of renal transplantation.
Crystalloids are classified into 2 large groups. Unbalanced and balanced crystalloids; the NS is considered unbalanced fluid.
The NS contains 154mEq/L of sodium and 154mEq/L of chloride; therefore it has no buffer capacity.
From the standpoint of renal hemodynamics, it tends to reduce the volume of diuresis, prolonging it over time. The activity of natriuretic factors, the inhibition of antinatriuretic system and the effect on cardiac output is similar to that of balanced solutions,24 but the water management is different from unbalanced crystalloid solution. With very large volumes of infusion and in the absence of spurious stimuli of ADH, it tends to produce hypernatremia. By contrast, the infusion of discreetly hypotonic solutions in large quantities favors hyponatremia more than hypernatremia.25,26 The relative hypotonicity of certain balanced crystalloids solutions causes inhibition of ADH and the water diuresis occurs earlier and more satisfactory than with NS.26 However, at this point it is important to remember that the inhibition of ADH release induced by resuscitation together with administration of hypoosmotic balanced solutions will promote the entry of water into the interstitial space, with the consequent deleterious effect that may occur in certain clinical circumstances.27
With regard to glomerular filtration, the infusion of NS, by distending the right cardiac cavities, increases the secretion of atrial natriuretic peptide, which dilates the afferent artery and inhibits the sodium channels of the collecting tubule. Therefore, the delay in the initiation of diuresis is a tubular effect, secondary to the activation of ADH due to a relative hypertonicity, so it requires a considerable volume of infusion.28
Hypovolemia due to situations as surgical interventions, forced diuresis, development of a third space or drainage, produce activation of the renin-angiotensin-aldosterone axis and increase in thirst.29 It should be remembered that the patient will develop hyponatremia if they are allowed to drink without salt, if we resuscitate with hypotonic solutions or glucose containing fluids without salt. Such salt depletion may increase the dependence of glomerular filtration on an intact renin-angiotensin system and sensitize the patient to the development of acute renal failure.30
Hyperchloremia, hyperchloremic metabolic acidosis and hyperkalemiaAccording to the Stewart model, the physical–chemical approach to the analysis of acid-base balance confers a predominant role to chloride, and hyperchloremia.31 The administration of fluids with supraphysiological concentrations of chloride and abnormal sodium-chloride concentration (NS) with respect to plasma will contribute to the development of hyperchloremic metabolic acidosis, since the relative increases in the concentration of chloride will cause a decrease in the difference of strong ions.32
Apart from the clinical effects of acidosis (reduction of cardiac contractility, reduction of catecholamine effects, alterations in coagulation or platelet function33), it is necessary to recall its effect on the regulation of serum potassium.
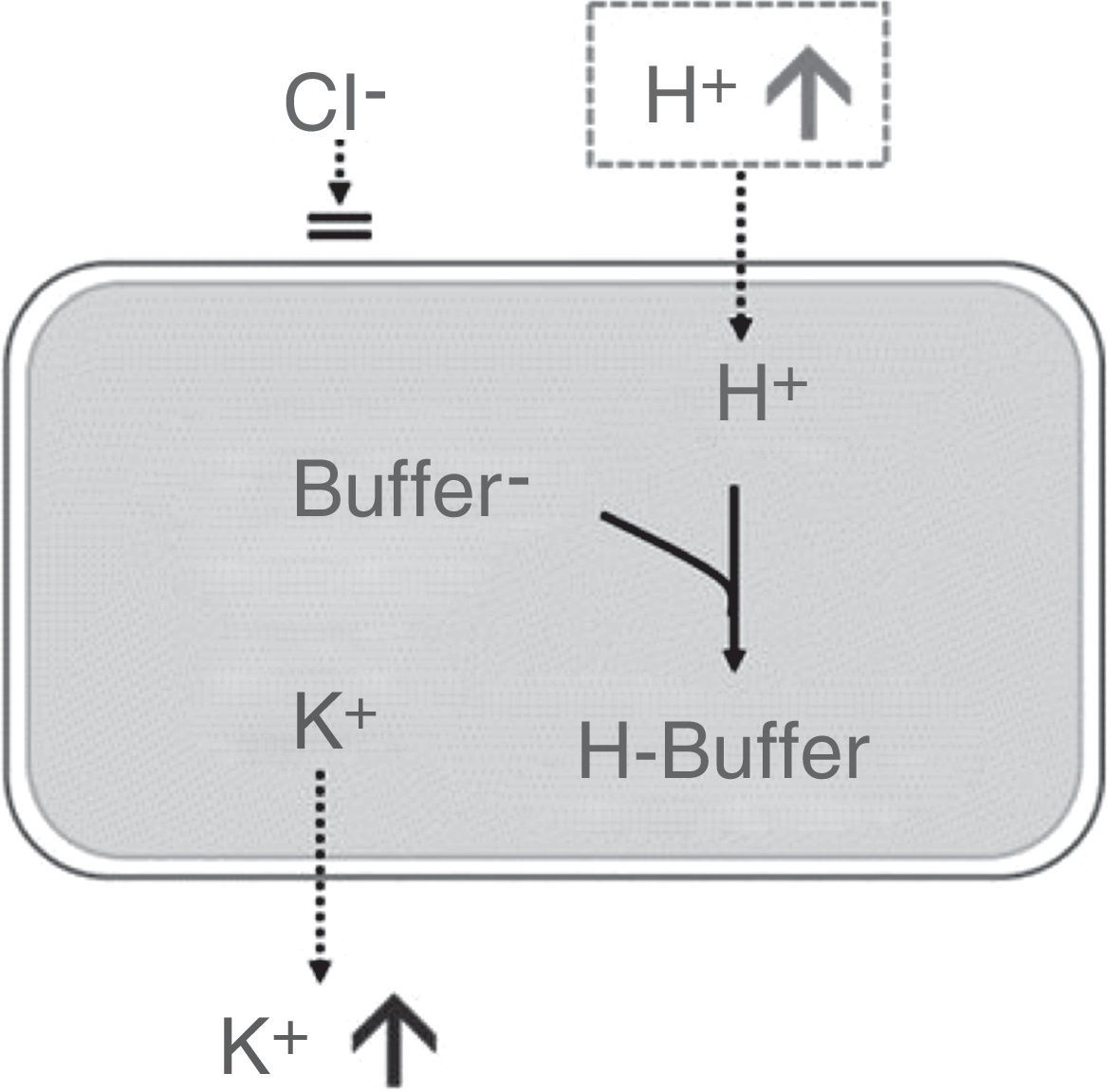
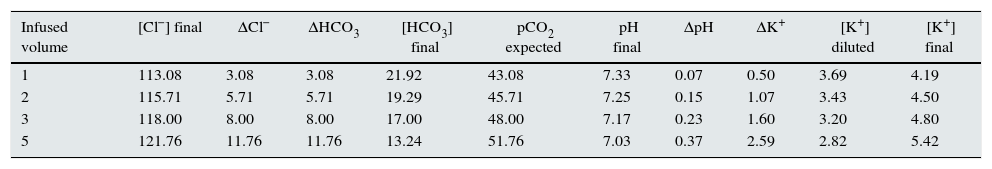
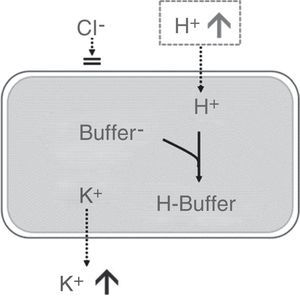
Approximately 98% of potassium is present within cells, with an intracellular concentration of ∼140mEq/L. Therefore, small changes in intracellular potassium will have a significant effect on extracellular potassium levels. In this context, hyperchloremic metabolic acidosis will cause the H+ ion to enter the cell to be neutralized with the intracellular buffer, with subsequent shifting of potassium outside the cells, which will increase the concentration of extracellular potassium34,35 (Fig. 1). Presumably, in a standard individual receiving a saline crystalloid con150mEq/L of chloride in its formulation, the serum potassium would increase above 5mEq/L for a volume infusion greater than 4L (see Table 1).
Effect of hyperchloremic acidosis on serum potassium concentration. Source: Modified from Santi et al.35 Transcellular shift of potassium driven by the entry of H+ into the cell where it is neutralized by intracellular buffers.
Simulation of the expected changes in the serum levels of chloride, bicarbonate, pCO2, pH and potassium of a standard subject treated with progressive volume expansion.
| Infused volume | [Cl−] final | ΔCl− | ΔHCO3 | [HCO3] final | pCO2 expected | pH final | ΔpH | ΔK+ | [K+] diluted | [K+] final |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 113.08 | 3.08 | 3.08 | 21.92 | 43.08 | 7.33 | 0.07 | 0.50 | 3.69 | 4.19 |
| 2 | 115.71 | 5.71 | 5.71 | 19.29 | 45.71 | 7.25 | 0.15 | 1.07 | 3.43 | 4.50 |
| 3 | 118.00 | 8.00 | 8.00 | 17.00 | 48.00 | 7.17 | 0.23 | 1.60 | 3.20 | 4.80 |
| 5 | 121.76 | 11.76 | 11.76 | 13.24 | 51.76 | 7.03 | 0.37 | 2.59 | 2.82 | 5.42 |
It is assumed 12L of extracellular volume for a 70kg subject. With a starting serum chloride level of 110mEq/L and potassium of 4mEq/L. It is infused a hypothetical saline crystalloid with a chloride concentration of 150mEq/L.
In the case of infusion of balanced solutions with buffer capacity, the presence of lactate or acetate will result in equivalent amounts of bicarbonate, which will prevent or minimize hyperchloremic acidosis. In fact, if such buffered solutions did not contain potassium in their formulation, the dilution effect on serum potassium concentration would cause hypokalemia by dilution.
At this point, it is important to note that the theoretical pathophysiological models support that the metabolism of lactate and acetate to achieve the production of bicarbonate differ in several aspects. First, it is considered that the production of bicarbonate from acetate is faster, with less oxygen consumption and not dependent exclusively on hepatic metabolism. Second, it does not interfere with gluconeogenesis and it is not a marker of tissue hypoxia, unlike lactate.36,37 In this sense, an observational study carried out on burn patients, comparing both buffer solutions, showed that in the first 5 days the serum lactate concentrations were significantly lower in the acetate group, with values of excess of bases significantly lower, although within the normal limits. The authors hypothesized that these high values of lactate with a normalization of excess bases were interpreted as being produced by the composition of the solution infused: they considered that these values of “iatrogenic” lactate was the cause of a greater infusion of fluids if there were not properly interpreted.36
Presence of calcium in resuscitation solutionsThe presence of calcium in the fluids may be responsible for significant clinical differences.
The effect of saline versus a calcium-balanced crystalloid was compared in an animal model subjected to uncontrolled bleeding produced by similar vascular lesions and with a therapeutic objective of maintaining a stable blood pressure. It was observed that the therapeutic goal was achieved with less volume infusion of the balanced crystalloid. Bleeding animals receiving saline have more blood losses than those treated with calcium. It is evident that the presence calcium modified the hemostasis of the animals. And, reasonably, the volume of diuresis was related to the volumes infused as the blood pressure remained constant.38
However, other authors have criticized this presence of calcium in some balanced crystalloids, arguing that they can facilitate microthrombi if they are used in large quantities in patients receiving multiple transfusions, since calcium antagonizes the effect of citrate.39
The choice of fluids in the perioperative renal transplant in clinical practiceThe perioperative period in renal transplantation has traditionally been a period of time in which large amounts of resuscitation fluids are administered, with the ultimate aim of ensuring the function of the graft after renal transplantation.1,8,10 The selection of patients who required a limited amounts fluid for resuscitation has been one of the criticisms of large studies, which sought to find differences in the renal protection exerted by the balanced solutions against NS.40,41
In the last decade, several studies have compared changes the ionic and acid–base produced by different crystalloids administration during the perioperative period of renal transplantation. After searching the databases Medline, Embase, Cochrane Database and Lilacs, we describe, according to year of publication, the most relevant studies in this regard:
O’Malley CM et al., year 2005: A randomized, double-blind comparison of lactated Ringer's solution and 0.9% NaCl during renal transplantation.7
Study carried out in 51 patients including live donors or cadaveric transplants. Exclusion criteria were serum potassium levels >5.5mEq/L pre-surgery. Twenty-five patients were randomized to the balanced crystalloid group and 26 to the NS group. The primary endpoint of the study was to determine differences in serum creatinine on the third postoperative day.
The mean creatinine value (mg/dL) on day 3 was 2.3±1.8 in the balanced crystalloid group versus 2.1±1.7 in the NS group (with no statistical significance). Five patients (19%) in the NS group versus zero patients (0%) in the balancing group had potassium concentrations ≥6mEq/L and the hyperkalemia had to be treated (p=0.05). Eight patients (31%) in the NS group versus zero patients (0%) in the balanced crystalloid group were treated for metabolic acidosis (p=0.004).
Hadimioglu et al., year 2008: The effect of different crystalloid solutions on acid–base balance and early kidney function after kidney transplantation.10
In this double-blind study, patients were randomly assigned to 3 groups (n=30) to receive NS, lactated Ringer's (RL), or Plasmalyte at doses of 20–30mL/kg. All 90 patients received live donor organ. Exclusion criteria was serum potassium levels >5.5mEq/L pre-surgery. The primary objectives of the study were to analyze: total daily urinary volume, serum creatinine on the third postoperative day, pH, bicarbonate and potassium levels during surgery and in the postoperative period, as well as creatinine, BUN, chloride, urinary output and creatinine clearance on days 1, 2, 3 and 7.
Results showed a statistically significant reduction in pH, in excess of alkali and a significant increase in serum chloride levels in patients receiving NS during surgery. Potassium levels did not show significant changes in any group. Measured in mM/L, the chloremia ranged between 21.2 of NS, 3.3 for RL and 1.7 in Plasmalyte.
Khajavi et al., year 2008: Effects of normal saline vs. Lactated Ringer's during renal transplantation.8
Randomized, double-blind study conducted in 52 patients with live donor grafts. Patients with serum potassium values ≥6mEq/L pre-surgery were excluded. The primary objectives were to find differences in serum potassium and pH at the end of surgery. The infusion fluids were administered at 60mL/kg according to protocol to maintain central venous pressure between 10 and 15mmHg.
The authors found hyperkalemia and acidosis more frequently in the NS group, showing a significant difference in serum potassium levels (p=0.000) and in pH (p=0.007).
Modi et al., year 2012: A comparative study of impact of infusion of Ringer's lactate solution versus normal saline on acid-base balance and serum electrolytes during live related renal transplantation.9
Randomized study, carried out in 74 patients (37 patients per arm) receiving infusion of RL solution versus NS; exclusion criteria was a serum potassium level ≥5.5mEq/L pre-surgery. The primary objectives were to compare urinary output intraoperatively and during the first postoperative day, serum creatinine values on the first postoperative day, changes in pH, bicarbonate, potassium and chloride during surgery and in the postoperative period. The anesthesia protocol was to maintain the central venous pressure between 12 and 15mmHg.
The volume administered in both groups during surgery was similar (RL=5.25L; NS=5.1L). The pH decreased from 7.43 to 7.33 in patients receiving NS and no pH changes were observed in the RL group. The mean value of serum creatinine on the first day after surgery was 2.43±0.87mg/dL in the RL group and 2.82±0.75mg/dL in the NS group. The serum potassium reached 3.99±0.71 versus 4.31±0.05 in the NS group (p<0.05). The serum chloride level was 98.50±3.03 versus 103.92±4.28 in the NS group (p<0.05).
Kim et al., year 2013: Comparison of the effects of normal saline versus Plasmalyte on acid–base balance during living donor kidney transplantation using the Stewart and base excess methods.42
A double-blind study in which patients were randomized, on the day before surgery, to NS group (n=30) or to Plasmalyte (n=30). In 100% of the cases there were living donors. The fluids were administered to maintain central venous pressure between 12 and 15mmHg. A total of 750mL of 5% albumin was given to all patients during surgery.
Arterial blood samples were collected after induction of anesthesia (T0), immediately before the anastomosis of the iliac vein (T1), 10min after reperfusion (T2) and at the end of surgery (T3) to measure pH, PaCO2, excess bases, bicarbonate, sodium, potassium, chloride, lactate, phosphate and albumin. The water balance was calculated during the study, as well as serum levels of chloride and creatinine at 24h and at days 1, 2 and 7. The acid-base state was analyzed using the physicochemical model of Stewart.
Chloride concentrations were significantly higher in T1, T2 and T3 in NS as compared to the Plamalyte group. None of the groups showed significant changes in serum K+ levels during surgery.
Postoperative serum chloride levels were not different between the 2 groups. Serum creatinine and 24-h urine volume were similar between groups.
Potura et al., year 2015: An acetate-buffered balanced crystalloid versus 0.9% saline in patients with end-stage renal disease undergoing cadaveric renal transplantation: a prospective randomized controlled trial.11
A prospective, randomized, controlled trial including 150 patients, 74 received NS and 76 a crystalloid solution balanced with acetate during and after renal cadaver transplantation. Study fluids were administered at a rate of 4mL/kg/h (according to ideal body weight) during surgery, and at 2mL/kg/h after surgery and during the postoperative follow up period. Exclusion criteria were potassium levels >5.5mEq/L pre-surgery.
The incidence of hyperkalemia differed between 17% and 21% (p=0.56) and the mean serum potassium variation since the initiation of surgery to the end of the study period was similar (mEq/L) (0.8 [0.0–1.0] versus 0.6 [0.0–1.0]; p=0.44).
Maximum serum chloride levels were significantly higher in the NS group (109mmol/L [107–111] versus 107mmol/L [105–109]). There was a significant trend toward developing hyperchloremia in the NS group as compared to the balanced crystalloid group (p=0.02).
More patients in the saline group, compared to the balancing group, required the administration of catecholamines for circulatory support. The difference was statistically significant.
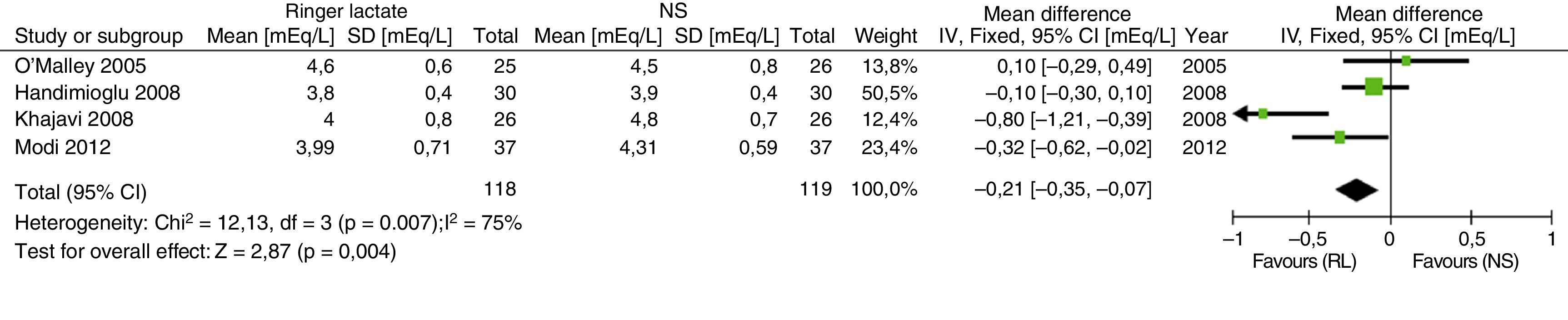
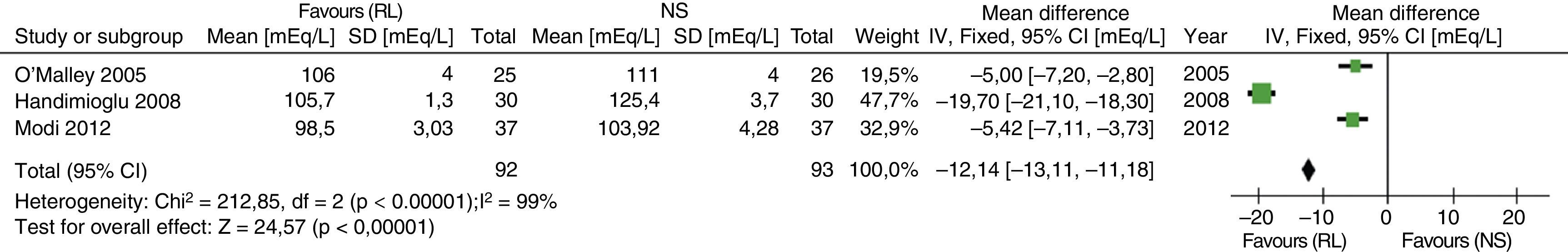
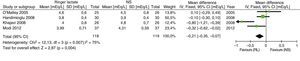
Recently, the results of a meta-analysis with 4 of the previously described articles have been published. SELECTION CRITERIA: Randomized controlled trials were included in adult renal transplant patients who compared the safety of RL versus NS.43 The results in relation to the development of hyperkalemia and hyperchloremia are shown in Figs. 2 and 3.
Results of the comparative meta-analysis of the Ringer's solution and the SSN, in relation to the development of hyperkalemia. Source: Taken from Trujillo-Zea et al.43
Results of the comparative meta-analysis of the Ringer's solution and the NS, in relation to the development of hyperchloremia. Source: Taken from Trujillo-Zea et al.43
First, despite the results of the studies described above, it should be emphasized that number of studies presently available is not sufficient to make a entirely correct choice of fluids in the perioperative period of renal transplantation. In addition, the studies described so far include a low number of patients, with different periods of observation and post-transplant follow-up and the variables evaluated are not always the same; these are premises that limit the interpretation of the data.
However, it can be concluded that the use of balanced crystalloids that include potassium in their formulation, during the perioperative period of renal transplantation, appear to be safe with respect to the control of serum potassium concentration. In addition, with the use balanced solutions there seems to be a better control of acid-base balance. No significant changes in serum creatinine have been observed during the perioperative period, neither at 3 nor at 7 days.
Key concepts
- •
The use of balanced crystalloids containing potassium in their formulation, during the perioperative period of renal transplantation, does not cause a greater alteration of serum potassium than that observed with NS.
- •
Hyperchloremia caused by infusion of NS causes hyperchloremic metabolic acidosis.
- •
Hyperchloremic metabolic acidosis may favor an increase in serum potassium concentration.
- •
It is necessary to perform clinical studies in these patients, in which variables of a greater clinical impact are evaluated.
The corresponding author, Dr. González-Castro, declares as potential conflict of interest collaborative work with the pharmaceutical company Baxter.
The rest of the authors declare no conflict of interest.
Please cite this article as: Gonzalez-Castro A, Ortiz-Lasa M, Peñasco Y, González C, Blanco C, Rodriguez-Borregan JC. Elección de fluidos en el periodo perioperatorio del trasplante renal. Nefrologia. 2017;37:572–578.