Intradialytic hypotension (IDH) is a common complication and is associated with higher morbidity and mortality in patients on haemodialysis (HD). However, there is a lack of uniformity in definitions of IDH. The main objective of this study is to analyse clinical and dialysis related factors with several IDH definitions, and its relationship with morbidity and mortality in a cohort of HD patients.
MethodsObservational study with a 30-month follow-up period that includes 68 prevalent patients on HD with at least six months of treatment. We analysed 18 non-consecutive dialysis sessions (first three of each month of a six-month period), and different definitions of IDH were recorded. A positive event of IDH was defined if any definition occurred in more than 25% of the sessions studied. Using survival analysis, we analysed the prediction capacity of each IDH definition (Nadir90, Nadir100, Fall20, Fall30, Fall20Nadir90, Fall30Nadir90, KDOQI, HEMO). The relationship with non-fatal cardiovascular disease (CVD) and global mortality was estimated using different Cox proportional models.
ResultsWe found IDH definitions that occurred significantly more frequently (Nadir100: 339.8/1000 sessions, Nadir90: 172.3/1000 sessions) than others (KDOQI: 98/1000 sessions, HEMO 129.9/1000 sessions). We registered 13 fatal events with a mean follow-up of 27.12 ± 6.84 months. A greater number of sessions with IDH according to the Nadir90 definition was a predictive factor of mortality (Log rank 5.02, p 0.025), independent according to adjusted models (HR: 3.23 [95% CI: 1.08–9.6], p 0.035). The definitions Nadir100 (HR: 4.54 [95% CI: 1.25−16.4], p 0.02) and Fall30Nadir90 (HR: 3.08 [95% CI: 1.07–8.8], p 0.03) were independent predictors of non-fatal CVD in adjusted models.
ConclusionIntradialytic hypotension, even asymptomatic, is a predictor of mortality and non-fatal cardiovascular disease in prevalent patients on haemodialysis.
La hipotensión arterial intradiálisis (HAID) es una complicación frecuente asociándose a una mayor morbimortalidad en hemodiálisis (HD), aunque es una tarea pendiente la uniformidad de criterios respecto a su definición. El objetivo del estudio es analizar las características de distintas definiciones de hipotensión, y su relación con la morbimortalidad en una cohorte de pacientes en HD.
MetodologíaEstudio observacional, con un seguimiento de 30 meses que incluye 68 pacientes prevalentes en hemodiálisis, con al menos 6 meses de tratamiento. Se recogieron parámetros de diálisis, y distintas definiciones de hipotensión, de 18 sesiones no consecutivas (las primeras 3 sesiones de cada mes de un período de 6 meses). Se definió como evento positivo de HAID si ocurría cualquier definición en más del 25% de las sesiones estudiadas. Se analizó el poder predictivo para cada definición de hipotensión (Nadir90, Nadir100, Fall20, Fall30, Fall20Nadir90, Fall30Nadir90, KDOQI, HEMO) mediante un análisis de supervivencia. Se estimó la relación con los eventos cardiovasculares (ECV) no fatales y la mortalidad global mediante distintos modelos proporcionales de Cox.
ResultadosEncontramos definiciones de HAID que ocurrieron con una significativa mayor frecuencia (Nadir100: 339,8/1.000 sesiones, Nadir90: 172,3/1.000 sesiones) en comparación con otras (KDOQI: 98/1.000 sesiones, HEMO 129,9/1.000 sesiones). Con una media de seguimiento de 27,12 ± 6,84 meses se registraron 13 eventos mortales. Un mayor número de sesiones con HAID acorde a la definición Nadir90 fue un factor predictor de mortalidad (Log rank 5,02, p 0,025), independiente según los modelos ajustados (HR: 3,23 [IC95%: 1,08-9,6], p 0,035). Las definiciones Nadir100 (HR: 4,54 [IC95%: 1,25-16,4], p 0,02) y Fall30Nadir90 (HR: 3,08 [IC95%: 1,07-8,8], p 0,03) fueron predictores independientes de ECV no fatales en los modelos ajustados.
ConclusionesLa hipotensión intradiálisis, incluso asintomática, tiene poder predictivo de mortalidad y eventos cardiovasculares no fatales en pacientes prevalentes en hemodiálisis.
Intradialytic hypotension (IDH) is a common complication of haemodialysis (HD), the prevalence is 5–30%.1 This wide range is due to a lack of uniformity in definitions of hypotension used across different studies.2 It was recently demonstrated that nadir-based definitions are more consistently associated with mortality in predictive terms3 as compared to traditional definitions, such as those proposed by the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines4 or the European Renal Best Practice Guidelines.5 In addition to differences in prevalence, IDH usually presents high variability between patients,1,6 which makes the question of what definition of intradialytic hypotension to use even more difficult to answer.
The high variability of blood pressure resulting from HD makes it hard to stablish objective of blood pressure control.7 Blood pressure tends to be higher in predialytic periods, it decreases during the dialysis procedure and increases again in the next interdialytic period.2 This variability is more marked in patients with conventional regimens (three sessions per week), since those with more intensive dialysis regimens such as daily HD have better blood pressure control without requiring antihypertensive agents.7 There are factors dependent on the individual patient that may influence a predisposition to episodes of intradialysis hypotension, these include capacity of cardiac response during ultrafiltration (UF) and residual renal function (RRF).8
The objective of the study was to analyse the clinical and dialysis-related characteristics of different definitions of IDH and the relationship with mortality in a cohort of HD patients.
MethodologyStudy designThis was an observational study with a follow-up period of 30 months that evaluated clinical features and characteristics related to dialysis sessions in 68 prevalent patients on HD. The following criteria were used to determine participation in the study. Inclusion criteria: prevalent patients on HD 18–80 years of age with at least six months of renal replacement therapy, clinical stability during the three months prior to the initiation of the study (defined as no hospital admission, surgeries or clinical events) who signed the informed consent form. Exclusion criteria: not willing to participate in the study and/or sign the informed consent. The study was conducted according to good clinical practice guidelines, and recruitment was started following the approval of the Research Ethics Committee.
Variables analysedThe demographic, clinical, laboratory and body composition variables collected were: age, gender, chronic kidney disease (CKD) aetiology, time on dialysis, prior transplants, history of atherosclerotic cardiovascular disease (ischaemic cardiomyopathy, peripheral vascular disease, mesenteric ischaemia) and non-atherosclerotic cardiovascular disease (heart failure, arrhythmias), vascular access, and body composition data obtained via multi-frequency bioimpedance spectroscopy analysis using a Body Composition Monitor (Fresenius Medical Care [FMC]) in the three months prior to the initiation of the study: overhydration (OH), total body water (TBW), extracellular water (ECW), lean body mass and body fat. Data on antihypertensive agents (number of drugs, class of antihypertensive agent: beta blockers, renin–angiotensin system blockers [RASBs], calcium channel blockers, diuretics, etc.) were also collected. The laboratory parameters collected were those related to anaemia (haemoglobin), bone mineral metabolism (calcium, phosphorus, parathyroid hormone [PTH]) and inflammation (high-sensitivity PCR).
Laboratory parameters were measured via standardised methods using autoanalysers. Serum PCR was measured via latex particle-enhanced turbidimetric immunoassay using a Hitachi analyser (Sigma Chemical Co., St. Louis, Missouri, United States).
Haemodialysis characteristicsA retrospective study was conducted on 18 non-consecutive sessions from a six-month period (first three sessions of each month) in all patients on the usual regimen. The dialysis modality, online haemodiafiltration [OL-HDF] or high-flow HD, and all prescription-specific parameters were selected at the clinician's discretion, according to the protocol of the hemodialysis unit :blood flow [Qb], dialysate flow [Qd], dialysate temperature [°C], conductivity [mSm/cm], dialysate composition. All patients were dialysed using a 5008 CorDiax monitor (FMC) on a regimen of three sessions per week of 240 min each. Different dialysers were used according to medical prescription (FXCorDiax80, FXCorDiax600, FXCorDiax800, FXCorDiax1000 [FMC], Polyflux 210H [Baxter]). The data collected in relation to dialysis sessions included: blood flow (Qb), dialysate temperature, Kt/V (Daugirdas's second-generation formula for single-pool), convective transport (litres/session), interdialytic weight gain (grams), UF rate (ml/h/kg), urea reduction ratio (RR) and ®2-microglobulin (RR [%]: 100 · [C1-C2]/C1, where C1 corresponds to initial serum concentration [pre-dialysis] and C2 corresponds to final serum concentration [post-dialysis]).
Changes in dry weight (session by session) were taken into consideration to obtaining UF rate per session. These data were used to calculate the UF rate (ml/kg/h); subsequently, the mean (of the UF rate) of the 18 sessions was estimated in each patient.
Definitions of IDHBlood pressure was monitored every half hour according to the protocol of the dialysis unit, using the automated system built into the 5008 CorDiax (FMC). The number of sessions fulfilling each definition of hypotension was verified. Mean episodes of hypotension (according to each definition) for the 18 sessions analysed per patient was calculated, and number of episodes of hypotension per 1000 dialysis sessions was also recorded as another measure of frequency. We defined a positive IDH event as fulfilment of the definition in more than 25% of the sessions analysed, and we obtained the proportion of patients who met this definition of a positive event for each definition of IDH used in the study.
The definitions of IDH used were those found in most reports of the literature3: Nadir90 (minimum intra-HD systolic blood pressure [SBP] <90 mmHg), Nadir100 [minimum intra-HD SBP < 100 mmHg], Fall20 ([pre-HD SBP – minimum intra-HD SBP]≥20 mmHg), Fall30 ([pre-HD SBP – minimum intra-HD SBP]≥30 mmHg), Fall20Nadir90 ([pre-HD SBP – minimum intra-HD SBP]≥20 mmHg and minimum intra-HD SBP < 90 mmHg), Fall30Nadir90 ([pre-HD SBP – minimum intra-HD SBP]≥30 mmHg and minimum intra-HD SBP < 90 mmHg), KDOQI ([pre-HD SBP – minimum intra-HD SBP]≥20 mmHg and symptoms that could be attributed to hypotension) and HEMO (any drop in SBP resulting in an intervention [reducing Qb or UF or administering saline]).
Cardiovascular events and mortalityNon-fatal and fatal cardiovascular events were collected with a follow-up period of 30 months from the start of the study. Cardiovascular events were defined as acute myocardial infarction (diagnosed by elevation of cardiac markers and changes on ECG and confirmed by cardiac catheterisation), heart failure [diagnosed by clinical criteria (Framingham) or an ejection fraction <45%], stroke (diagnosed by computed tomography), peripheral vascular disease (diagnosed by stenosis of a main artery of the lower limbs confirmed by angiography, need for amputation or any ischaemic condition affecting other regions such as mesenteric ischaemia or optic neuritis).
Statistical analysis of the dataContinuous variables were analysed using the Kolmogorov–Smirnov test for verification of a normal distribution. Those variables with a normal distribution are described in terms of mean ± SD; those with a non-normal distribution are described as median and interquartile range. Quantitative variables with a normal or a non-normal distribution were compared using the Pearson's or the Spearman's correlation test, respectively. Parametric data were compared using Student's test or analysis of variance (ANOVA). Categorical variables were expressed in terms of percentage and compared using the chi-square test. A survival analysis (Kaplan–Meier) was used to estimate predictive capacity for mortality of each definition of intradialytic hypotension. Finally, a Cox proportional hazards analysis was used to estimate the relationship between mortality and IDH. A p-value <0.05 with a 95% confidence interval was considered statistically significant. The analysis was performed using the SPSS software package, version 20.0 (SPSS, Inc., Chicago, IL, UnitedStates).
ResultsBaseline characteristicsThe most prevalent causes of CKD were diabetic nephropathy (22.1%) and glomerular nephropathy (22.1%); the least common cause was vascular nephropathy (5.9%). Around 60% of patients were on antihypertensive agents, with a mean number of drugs of 0.6±0.4. Beta blockers (35.3%), renin–angiotensin system blockers (26.5%) and diuretics (11.8%) were the most commonly used drugs. Baseline characteristics are summarised in Table 1.
Baseline characteristics.
| Clinical/laboratory findings | Mean ± SD n (%) |
|---|---|
| Age (years) | 58.6 ± 14.7 |
| Gender (male/female), n (%) | 44 (64.7) / 24 (35.3) |
| Diabetes mellitus, n (%) | 16 (23.5) |
| AVF/Catheter, n (%) | 59 (86.8) / 9 (13.2) |
| Cardiovascular disease, n (%) | 34 (50) |
| Time on HD (months)a | 46.5 (24−104) |
| Residual renal function, n (%)b | 18 (26.5) |
| OL-HDF, n (%) | 50 (73.5) |
| Patients on anti-HTN agents, n (%) | 43 (63.2) |
| Haemoglobin (g/dl) | 11.3 ± 1.3 |
| Serum calcium (g/dl) | 8.6 ± 0.6 |
| Serum phosphorus (mg/dl) | 3.9 ± 1.5 |
| C-reactive protein (CRP) (mg/dl) | 0.8 ± 1.4 |
| Serum albumin (g/dl) | 3.7 ± 0.4 |
| HDL cholesterol (mg/dl) | 46.2 ± 15.8 |
| BMI (kg/m2) | 24.9 ± 6.4 |
| Overhydration (l) | 1.47 ± 1.59 |
| Lean body mass (kg) | 35.1 ± 11.6 |
| Lean tissue index (kg/m2) | 12.8 ± 3.4 |
| Body fat mass (kg) | 28.3 ± 15.2 |
| Fat tissue index (kg/m2) | 12.3 ± 6.1 |
Body composition data were obtained using a Body Composition Monitor (FMC).
AVF: arteriovenous fistula; BMI: body mass index; HD: haemodialysis; HDL: high-density lipoprotein; HTN: hypertension; n: number of patients; OL-HDF: online haemodiafiltration; SD: standard deviation.
The main characteristics of the 1224 dialysis sessions (18 per patient) are summarised in Tables 2 and 3.
Characteristics of dialysis sessions.
| Parameters related to HD | Mean ± SD |
|---|---|
| Predialysis SBP (mmHg) | 139.2 ± 16.8 |
| Postdialysis SBP (mmHg) | 128.9 ± 17.2 |
| Interdialytic weight gain (g) | 1873 ± 685 |
| Ultrafiltration (ml) | 1866 ± 717 |
| Ultrafiltration rate (ml/h/kg) | 7.4 ± 2.9 |
| Number of sessions with ultrafiltration rate >12 mL/h/kg | 1.8 ± 3.1 |
| Kt/V | 2.0 ± 0.5 |
| Convective volume, patients on OL-HDF (l) | 27.6 ± 4.1 |
| Dialysate temperature (°C) | 35.6 ± 0.4 |
| Dialysate conductivity (mSm/cm) | 13.7 ± 0.1 |
| Urea reduction ratio (%) | 81.9 ± 6.7 |
| β2-microglobulin reduction ratio (%) | 79.1 ± 8.1 |
Kt/V: obtained by ionic dialysance using a 5008 monitor (FMC).
HD: haemodialysis; OL-HDF: online haemodiafiltration; SBP: systolic blood pressure.
Characteristics of dialysis sessions by dialysis modality.
| OL-HDF n = 50 | High-flow HD n = 18 | p | |
|---|---|---|---|
| Qb (ml/min.) | 463.3 ± 49.1 | 410.2 ± 67.9 | 0.006 |
| Dialysate temperature (°C) | 35.6 ± 0.4 | 35.5 ± 0.3 | ns |
| Kt/Vs | 2.0 ± 0.4 | 1.8 ± 0.5 | ns |
| Convective volume, OL-HDF (l/session) | 27.6 ± 4.1 | – | – |
| β2-microglobulin RR (%) | 80.2 ± 6.5 | 70.5 ± 13.7 | 0.010 |
| Urea RR (%) | 82.1 ± 6.6 | 81.2 ± 8.5 | ns |
| IDWG (g) | 1,917.6 ± 694.3 | 1,749.6 ± 661.5 | ns |
| Ultrafiltration (ml) | 1,914.1 ± 736.4 | 1,732.4 ± 661.3 | ns |
| UF rate (ml/h/kg) | 7.5 ± 3.2 | 7.1 ± 1.9 | ns |
| Predialysis SBP (mmHg) | 139.2 ± 18.3 | 139.2 ± 12.4 | ns |
| Postdialysis SBP (mmHg) | 129.0 ± 19.0 | 128.7 ± 11.2 | ns |
Kt/V: obtained by ionic dialysance using a 5008 monitor (FMC).
IDWG: interdialytic weight gain; HD: haemodialysis; OL-HDF: online haemodiafiltration; ns: not significant; Qb: blood flow; RR: reduction ratio; SBP: systolic blood pressure; UF: ultrafiltration.
The most common definitions in the study were "asymptomatic" (Fall20: 11.0 ± 4.2 sessions; Fall30: 7.9 ± 4.8 sessions; Nadir90: 3.1 ± 3.8 sessions; Nadir100: 6.1 ± 5.7 sessions) versus "symptomatic" (KDOQI: 1.7 ± 2.0 sessions; HEMO: 2.3 ± 2.5 sessions) (Table 4).
Prevalence of definitions of intradialytic hypotension.
| IDH definition | n/1000 sessions | Patients with a positive IDH event (>25% of sessions) |
|---|---|---|
| Nadir90 | 172.3 | 27.9% |
| Nadir100 | 339.8 | 50% |
| Fall20 | 611.1 | 95.6% |
| Fall30 | 441.1 | 73.5% |
| Fall20Nadir90 | 145.4 | 23.5% |
| Fall30Nadir90 | 120.1 | 16.2% |
| KDOQI | 98.0 | 10.3% |
| HEMO | 129.9 | 20.6% |
A positive IDH event was defined as fulfilment of a definition in >25% of the sessions analysed.
IDH: intradialytic hypotension.
No differences were observed between rate of IDH (all definitions) and presence of RRF. However, we did find that patients with RRF required a significantly lower UF rate (5.2 ± 2.5 mL/h/kg) than patients without RRF (8.2 ± 2.6 mL/h/kg) (p < 0.001). We also did not find any differences between IDH and history of cardiovascular disease, dialysis modality (OL-HDF or high-flow HD) or type of vascular access used for dialysis (arteriovenous fistula [AVF] versus catheter). The factors related to the different definitions of IDH are summarised in Table 5.
Factors related to intradialytic hypotension.
| [0,2,3Nadir90 | [0,4–5]Nadir100 | [0,6–7]KDOQI | [0,8–9]HEMO | |||||
|---|---|---|---|---|---|---|---|---|
| <25% sessions | >25% sessions | <25% sessions | >25% sessions | <25% sessions | >25% sessions | <25% sessions | >25% sessions | |
| Age (years) | 57.6 ± 13.7ns | 61.4 ± 17.2ns | 54.5 ± 13.6p = 0.019 | 62.8 ± 14.8p = 0.019 | 58.5 ± 15.4ns | 59.7 ± 6.8ns | 58.8 ± 14.6ns | 58.1 ± 15.7ns |
| Diabetes mellitus (%) | 24.5ns | 21.1ns | 23.5ns | 23.5ns | 19.7p = 0.048 | 57.1p = 0.048 | 20.4ns | 35.7ns |
| Time on HD (months) | 66.7 ± 70.1p = 0.043 | 109.3 ± 90.4p = 0.043 | 65.6 ± 63.7ns | 91.6 ± 89.6ns | 79.9 ± 81.0ns | 67.7 ± 48ns | 83.5 ± 83.8ns | 59.7 ± 47.6ns |
| Patients on antihypertensive agents (%) | 75.5p = 0.002 | 31.6p = 0.002 | 76.5p = 0.024 | 50p = 0.024 | 67.2ns | 28.6ns | 68.5ns | 42.9ns |
| Predialysis SBP (mmHg) | 143.8 ± 14.9p <0.001 | 127.4 ± 16.1p <0.001 | 147.6 ± 14.1p <0.001 | 130.9 ± 15.4p <0.001 | 138.8 ± 17.2ns | 143.9 ± 13.7ns | 139.6 ± 17.0ns | 137.8 ± 16.9ns |
| IDWG (g) | 1809 ± 690ns | 2038 ± 685ns | 1812 ± 783ns | 1934 ± 575ns | 1844 ± 678ns | 2123 ± 740ns | 1823 ± 701ns | 2063 ± 604ns |
| Ultrafiltration rate (ml/h/kg) | 7.1 ± 2.9ns | 8.2 ± 2.7ns | 7.0 ± 3.4ns | 7.8 ± 2.3ns | 7.2 ± 2.8ns | 9.4 ± 3.2ns | 7.1 ± 2.8ns | 8.4 ± 3.1ns |
| Predialysis serum β2-microglobulin (mg/l) | 20.9 ± 5.3p <0.001 | 26.4 ± 5.3p <0.001 | 20.3 ± 5.8p = 0.002 | 24.6 ± 5.2p = 0.002 | 22.2 ± 5.6ns | 25.1 ± 6.7ns | 22.2 ± 5.8ns | 23.6 ± 6.2ns |
| Drop in BV (%)a | 11.2 ± 3.8ns | 12.1 ± 2.8ns | 10.8 ± 4.2ns | 12.1 ± 2.8ns | 11.3 ± 3.3ns | 13.1 ± 4.3ns | 10.8 ± 3.3p = 0.03 | 13.5 ± 3.1p = 0.03 |
| Overhydration (l) | 1.5 ± 1.3ns | 1.3 ± 2.1ns | 1.6 ± 1.1ns | 1.2 ± 1.9ns | 1.4 ± 1.6ns | 1.7 ± 1.0ns | 1.4 ± 1.7ns | 1.5 ± 1.1ns |
| Kt/V | 1.9 ± 0.5ns | 2.0 ± 0.3ns | 2.0 ± 0.5ns | 1.9 ± 0.4ns | 1.9 ± 0.5ns | 2.1 ± 0.5ns | 2.0 ± 0.5ns | 1.9 ± 0.4ns |
A positive IDH event was defined as fulfilment of a definition in >25% of the sessions analysed.
Kt/V determined by ionic dialysance.
BV: blood volume; HD: haemodialysis; IDH: intradialytic hypotension; IDWG: interdialytic weight gain; ns: not significant; SBP: systolic blood pressure.
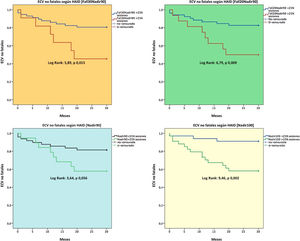
A total of 17 non-fatal cardiovascular events were recorded with the following distribution: heart failure (n = 8), ischaemic cardiomyopathy (n = 5), stroke (n = 2), peripheral vascular disease (n = 2). A higher number of IDH sessions was a predictive factor for these non-fatal events (Nadir100, Fall20Nadir90, Fall30Nadir90 [Fig. 1]), and the Nadir100 and Fall30Nadir90 definitions were predictive factors independent of non-fatal cardiovascular events in the adjusted models (Table 6).
Multivariate analysis for non-fatal cardiovascular events.
| [1,0]Model 1 | [0,2–4]Multivariate analysis | ||
|---|---|---|---|
| HR | (95% CI) | p | |
| Age | 1.00 | (0.96−1.04) | 0.6 |
| Sex (male ref.) | 0.32 | (0.092−1.15) | 0.08 |
| Time on HD | 1.00 | (0.99−1.00) | 0.3 |
| IDH (Nadir100) | 4.54 | (1.25−16.4) | 0.02 |
| CVDH | 4.01 | (1.03−15.52) | 0.04 |
| Diabetes mellitus | 0.62 | (0.17−2.28) | 0.4 |
| [1,0]Model 2 | [0,2–4]Multivariate analysis | ||
|---|---|---|---|
| HR | (95% CI) | p | |
| Age | 1.02 | (0.98−1.06) | 0.2 |
| Sex (male ref.) | 0.34 | (0.09−1.21) | 0.09 |
| Time on HD | 1.00 | (0.99−1.01) | 0.1 |
| IDH (Fall30Nadir90) | 3.08 | (1.07−8.87) | 0.03 |
| CVDH | 2.97 | (0.73−12.08) | 0.1 |
| Diabetes mellitus | 0.66 | (0.18−2.46) | 0.6 |
| [1,0]Model 3 | [0,2–4]Multivariate analysis | ||
|---|---|---|---|
| HR | (95% CI) | p | |
| Age | 1.01 | (0.97−1.06) | 0.4 |
| Time on HD | 1.00 | (0.99–1.01) | 0.07 |
| IDH (Nadir100) | 1.70 | (0.42−6.91) | 0.4 |
| β2-microglobulin RR | 0.92 | (0.85−0.99) | 0.03 |
| [1,0]Model 4 | [0,2–4]Multivariate analysis | ||
|---|---|---|---|
| HR | (95% CI) | p | |
| Age | 1.03 | (0.99−1.07) | 0.1 |
| Time on HD | 1.00 | (1.00−1.015) | 0.04 |
| IDH (Fall30Nadir90) | 4.27 | (1.26−14.47) | 0.02 |
| β2-microglobulin RR | 0.92 | (0.86−0.98) | 0.02 |
A positive IDH event was defined as fulfilment of a definition in >25% of the sessions analysed.
CI: confidence interval; CVDH: cardiovascular disease history (atherosclerotic or non-atherosclerotic origin); HD: haemodialysis (time on haemodialysis in months); HR: hazard ratio; IDH: intradialytic hypotension; RR: reduction ratio.
A total of 13 fatal events were recorded in a mean follow-up period of 27.12 ± 6.84 months. The causes of mortality were infection (38.5%), cardiovascular disease (30.8%), sudden death (7.7%) and other causes (23.1%, notably including tumours).
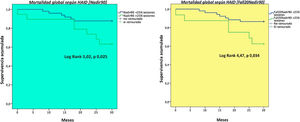
A higher number of sessions with IDH (Nadir90 and Fall20Nadir90 definitions) was a predictor of mortality (Fig. 2). Hypotension, according to the Nadir90 definition, was an independent predictive factor for mortality at 30 months, whereas other factors such as advanced age and a low predialysis SBP were also independent predictors of mortality. We also found that OL-HDF was associated with a lower rate of mortality during follow-up (Table 7).
Multivariate analysis for all-cause mortality.
| [1,0]Model 1 | [0,2–4]Multivariate analysis | ||
|---|---|---|---|
| HR | (95% CI) | p | |
| Age | 1.04 | (0.99−1.09) | 0.1 |
| Sex (male ref.) | 0.56 | (0.15−2.07) | 0.3 |
| Time on HD | 0.99 | (0.99−1.01) | 0.7 |
| IDH (Nadir90) | 3.2 | (1.08−9.64) | 0.035 |
| CVDH | 0.72 | (0.17−3.14) | 0.6 |
| Diabetes mellitus | 0.46 | (1.01−2.18) | 0.3 |
| [1,0]Model 2 | [0,2–4]Multivariate analysis | ||
|---|---|---|---|
| HR | (95% CI) | p | |
| Age | 1.04 | (1.00−1.08) | 0.03 |
| Sex (male ref.) | 0.58 | (0.15−2.2) | 0.4 |
| Time on HD | 0.99 | (0.99−1.06) | 0.6 |
| IDH (Fall20Nadir90) | 1.9 | (0.53−7.06) | 0.3 |
| CVDH | 1.3 | (0.39−4.8) | 0.6 |
| Diabetes mellitus | 0.47 | (0.09−2.2) | 0.3 |
| [1,0]Model 3 | [0,2–4]Multivariate analysis | ||
|---|---|---|---|
| HR | (95% CI) | p | |
| Age | 1.02 | (0.99−1.07) | 0.2 |
| HD modality (OL-HDF ref.) | 0.26 | (0.07−0.92) | 0.03 |
| IDH (Nadir90) | 1.41 | (0.37−5.3) | 0.6 |
| Predialysis SBP | 0.95 | (0.91−1.00) | 0.06 |
| [1,0]Model 4 | [0,2–4]Multivariate analysis | ||
|---|---|---|---|
| HR | (95% CI) | p | |
| Age | 1.02 | (0.98−1.07) | 0.2 |
| HD modality (OL-HDF ref.) | 0.26 | (0.07−0.93) | 0.03 |
| IDH (Fall20Nadir90) | 1.2 | (0.34−4.3) | 0.7 |
| Predialysis SBP | 0.95 | (0.91−0.99) | 0.03 |
A positive IDH event was defined as fulfilment of a definition in >25% of the sessions analysed.
CI: confidence interval; CVDH: cardiovascular disease history (atherosclerotic or non-atherosclerotic origin); HD: haemodialysis (time on haemodialysis in months); HR: hazard ratio; IDH: intradialytic hypotension; OL-HDF: online haemodiafiltration; SBP: systolic blood pressure.
In a cohort of prevalent HD patients, we have demonstrated the power of IDH to predict non-fatal cardiovascular events and of overall mortality, especially with asymptomatic (nadir-based) definitions. This magnifies the importance of an episode of hypotension on dialysis regardless of whether or not it is accompanied by associated symptoms.
Suitable evaluation of the impact of IDH depends largely on the use of a definition that encompasses various clinical scenarios and approximates regular practice in a dialysis session as closely as possible. Some definitions are based on the need for implementing corrective measures such as reducing the UF rate and administering a saline solution, whereas other definitions are based on an absolute drop in SBP compared to predialysis SBP or are nadir-based definitions without the presence of symptoms.1,2 However, clinical guidelines use a combination of these factors, i.e. a drop in SBP accompanied by a clinical event plus the need for intervention.1,2 For example, the KDOQI guidelines and the European good practice guidelines define IDH as a drop in SBP greater than or equal to 20 mmHg, or a drop in mean BP of 10 mmHg, plus the presence of symptoms of hypotension (headache, cramps, chest pain, decreased visual acuity or any symptom that could be attributed to a situation of tissue hypoperfusion), requiring or not intervention measures to increase blood pressure or improve associated symptoms.3,4
This study used the eight definitions of IDH most commonly found in the literature, including those recommended by European clinical guidelines and KDOQI guidelines.1–5 Recently, Flythe et al. showed the predictive power for mortality of the Nadir90 definition in patients with a predialysis SBP around 130 mmHg,3 a figure for predialysis SBP similar to that of our study, with the same result for predictive power for mortality for the Nadir90 definition. However, with the Nadir100 definition we did not find a relationship with mortality; this could be explained by the mean predialysis SBP in our series, among other factors. The predictive capacity of Nadir100 was more consistent in patients with a predialysis SBP in excess of 140 mmHg.3
After evaluation of the relationship between IDH and non-fatal cardiovascular events, it was observed that the definition of hypotension that maintained the most consistent prediction in the adjusted models was the combination of Nadir90 and a drop in predialysis SBP by 30 mmHg (Fall30Nadir90). It should be noted that the survival analyses showed that other definitions also have a predictive power for fatal and non-fatal cardiovascular events, although the adjusted models did not show their relationship to be as consistent as the Nadir90 and Fall30Nadir90 definitions, respectively. The low predictive capacity of symptomatic IDH in our study was surprising and might have been conditioned by the low prevalence of the KDOQI and/or HEMO definition.
Our data reveal the high variability of IDH between patients, this is similar other reports on IDH.6 Regarding the definition of symptomatic IDH (KDOQI), 39% of study patients did not have any session with symptomatic hypotension. By contrast, at least one episode of symptomatic hypotension was recorded in 60% of patients. Something similar occurred with nadir-based definitions, although a drop by 20 mmHg in SBP (Fall20) was a constant phenomenon, to such a point that just one of the patients in the study did not experience any episodes of hypotension according to this definition. We defined IDH as fulfilment of the definition of hypotension in more than 25% of the sessions analysed. This cut-off point was similar to that proposed by other authors.3 If this cut-off point is considered, definitions of symptomatic IDH and/or IDH requiring some sort of intervention had a prevalence of 10.3% and 20.6%, respectively. By contrast, the prevalence of Nadir100 was 50% versus 27.9% for Nadir90. These differences regarding the prevalence of IDH seen in our data are similar to those reported in other studies with an IDH prevalence of 5–30%, and largely depend on the definition used.1,2
IDH is determined by patient-specific factors (advanced age, diabetes, more time on HD, low predialysis SBP, heart failure, autonomic dysfunction) but also by HD-specific factors.8 At present, there is enough clinical evidence to characterise HD as a risk factor from a haemodynamic point of view, especially in a vulnerable vascular tree.9,10 The cumulative negative effect of haemodynamic stress resulting from HD may harm organ function.9,10 The best example of this clinical scenario is a disease known as stunned myocardium.11 A common condition in HD,12 it entails a loss of contractile function at the time when greater cardiac output is required to compensate for a reduction in blood pressure.9–12 In addition there is the usual compromise in CKD of adaptive mechanisms in the microcirculation, which is aggravated in the context of a greater requirement for oxygen as occurs in left ventricular hypertrophy.8
In our study, the presence of heart disease was only associated with a drop in predialysis SBP by 30 mmHg (Fall30), although advanced age and more time on HD were factors associated with a higher frequency of episodes of hypotension according to nadir-based definitions. Diabetes mellitus increased the likelihood of experiencing a symptomatic episode of hypotension (KDOQI). The negative effect from a haemodynamic point of view acts as an expansive phenomenon, to such a point that all body organs can be critically affected. Notably, recent reports at the brain level by Polinder-Bos et al. and Findlay et al. demonstrated a significant drop in cerebral blood flow during an HD session,13,14 a specular image of what occurs at the cardiac level, which the authors called "stunned brain".14
A drop in blood pressure on HD is not usually accompanied by a positive effect on the mortality observed in the general population,7 and this study found that a lower predialysis SBP increased the likelihood of experiencing hypotension according to nadir-based definitions. It also found that a lower predialysis SBP was a predictive factor independent of mortality; this finding was similar to other authors.15–20 Although we lack sufficient evidence to determine the optimum range of blood pressure on HD, a growing body of data suggests that the relationship between morbidity and mortality and blood pressure follows a J- or U-shaped curve.15–20 Li et al., Klassen et al., Zager et al. and Bansal et al. obtained results similar to those presented in this study with the common denominator of a higher risk of mortality in a situation of "normotension" or of hypotension in patients on HD with different ranges of predialysis SBP predictive of mortality (e.g. < 120 mmHg, <130 mmHg, <110 mmHg).16–19 It is likely that the paradoxic effect of this relationship is explained by greater vulnerability from a haemodynamic point of view in patients with heart disease,20 and in our study a predialysis SBP above 140 mmHg was associated with a lower mortality during follow-up.
The dialysis regimen of the study patients consisted of three sessions per week with an optimal dialysis dose in the 1224 dialysis sessions analysed, consistent with that recommended by dialysis suitability guidelines.21 Interdialytic weight gain was less than 2 kg, a typical mean in our setting,22 which explains the UF rate of 7.4 ± 2.9 mL/h/kg. This rate is considerably lower than the cut-off points of 10, 12 and 13 mL/h/kg proposed by other authors as risk factors for cardiovascular morbidity and mortality.23,24 However, it is above 6 mL/h/kg, which other authors have identified as the cut-off point where the linear relationship between mortality and UF rate begins.25 An interesting aspect of our data is that we did not find any differences between the UF rate and the different definitions of hypotension.
Uniform criteria with regard to the ideal range for blood pressure on HD have yet to be established in the field of nephrology. The KDOQI guidelines, with a grade C recommendation, define a target predialysis blood pressure as <140/90 mmHg and a target postdialysis blood pressure as <130/80 mmHg.7 Based on information drawn from observational studies, this practice could expose patients to a higher rate of intradialytic hypotension, with a resulting negative effect on morbidity and mortality.15–20 Therefore, clinical trials that more clearly define treatment objectives for blood pressure in patients on HD are needed.
A key consideration related to haemodynamic tolerability on HD is the estimated dry weight. This concept has evolved as has dialysis itself, representing a real challenge for the nephrologist given the poor precision of a physical examination. To date, it remains a matter of debate.26,27 According to the most classic definition, dry weight represents the state of "normohydration" or euvolaemia after the dialysis session; thus it was determined that a patient reached their dry weight when on dropping from that level they experienced an episode of IDH.22,26–28 Bioimpedance spectroscopy is a tool that may aid in estimating dry weight,27 although calculation of dry weight is more an art and a matter of trial and error than a mathematical formula, or the result of the application of a technique.22 According to this line of argument, other authors propose that a "permissive state of hypervolaemia" would ensure perfusion of different organs as it maintains a state of slightly increased extracellular volume, which they define as "functional dry weight", and would be a reasonable option to prevent damage caused by states of hypoperfusion in the context of IDH.27 Our results reveal that we found no significant differences between the different definitions of hypotension. This was particularly striking in symptomatic definitions but also striking in nadir-based definitions. Other factors may account for these data (e.g. a low UF rate), but it is necessary to stress the role of the nephrologist in "correctly" estimating dry weight on HD, whether to achieve the objective of a state of "normovolaemia" or in cases in which it is preferable to achieve that "permissive state of hypervolaemia" explained by other authors as a protective mechanism against tissue damage on dialysis.
This study was not free from limitations. For example, its small sample size resulted in a weaker statistical power and impeded our ability to generalise our results. Other critical matters could be the retrospective analysis of the dialysis sessions — although we included a reasonable number of sessions, which gives an overall idea of the situation of the patient on dialysis — and the observational nature of the study, which precludes demonstration of a causal relationship between hypotension and cardiovascular events.
In conclusion, IDH, even asymptomatic, may have power to predict f mortality and non-fatal cardiovascular events in prevalent HD patients. This highlights the need to focus the efforts on preventing haemodynamic stress in order to minimise tissue damage and the resulting negative effect on morbidity and mortality.
FundingThis study has received no specific funding from public, private or non-profit organisations.
AuthorsSC: study design, data collection, statistical analysis, literature review, manuscript drafting. AV: statistical analysis, manuscript review. NM: statistical analysis, manuscript review. LS: data collection, statistical analysis. SA: statistical analysis, manuscript review. JMLG: study design, manuscript review. JL: manuscript review.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Cedeño S, Vega A, Macías N, Sánchez L, Abad S, López-Gómez JM, et al. Definiciones de hipotensión intradiálisis con poderpredictivo de mortalidad en una cohorte de hemodiálisis. Nefrologia. 2020;40:402–412.