Resistant hypertension (RH) is a significant health problem with complex management. The aim of this study was to evaluate the risks and benefits of adding spironolactone to treat RH.
Material and methodsIn total, 216 patients with RH in whom spironolactone (12.5–25mg daily) was added as an antihypertensive were evaluated. One-hundred and twenty-five (125) were analyzed retrospectively and 91 prospectively. Blood pressure (BP) and laboratory parameters (serum creatinine [sCrea], estimated glomerular filtration rate [eGFR] and serum potassium [sK]) were analyzed at baseline and at 3–6–12 months after introducing spironolactone.
ResultsA change of systolic/diastolic BP (mean±standard deviation) of −10.9±2.7/−4.3±1.6mmHg at 3 months and −13.6±2.8/−6.0±1.6mmHg at 12 months; p<0.001 was observed. These values were confirmed with ambulatory-BP monitoring at 12 months. At 3 months, an increase in sCrea of 0.10±0.04mg/dL, a decrease in eGFR of −5.4±1.9ml/min/1.73m2 and an increase in sK of 0.3±0.1mmol/l; p<0.001 was observed for all cases. These changes were maintained after 12 months. There were no significant differences in changes of BP, sCrea, eGFR and sK between 3 and 12 months. Results of the retrospective and prospective cohorts separately were superimposable. In the prospective cohort, spironolactone was withdrawn in 9 patients (9.9%) because of adverse effects.
ConclusionsAfter 3 months with spironolactone, a decrease in BP associated with a decrease in the eGFR and an increase in sCrea and sK was observed. These changes were maintained at 12 months. Spironolactone is an effective and safe treatment for RH in patients with baseline eGFR ≥30ml/min/1.73m2.
La hipertensión arterial resistente (HTAR) supone un importante problema de salud de manejo complejo. Este trabajo evalúa los riesgos y beneficios de añadir espironolactona para tratar la HTAR.
Material y métodosSe evaluaron 216 pacientes con HTAR a quienes se añadió espironolactona (12,5-25mg/día) como antihipertensivo. Ciento veinticinco se analizaron retrospectivamente y 91 prospectivamente. Se analizaron parámetros de presión arterial (PA) y laboratorio (creatinina plasmática [Creap], filtrado glomerular [FGe] y potasio plasmático [Kp]) al momento basal y tras 3-6-12 meses con espironolactona.
ResultadosSe objetivó una variación de PA sistólica/diastólica (media± desviación estándar) de −10,9±2,7/−4,3±1,6mmHg a los 3 meses y −13,6±2,8/−6,0±1,6mmHg a los 12 meses; p<0,001. Valores confirmados mediante monitorización ambulatoria de PA a los 12 meses. A los 3 meses, la Creap incrementó 0,10±0,04mg/dl, el FGe disminuyó −5,4±1,9ml/min/1,73m2 y el Kp incrementó 0,3±0,1mmol/l; p<0,001 para todos los casos. Estas variaciones se mantuvieron a los 12 meses. No hubo diferencias significativas en las variaciones de PA, Creap, FGe y Kp entre los 3 y 12 meses. Los resultados al analizar las cohortes retrospectiva y prospectiva por separado fueron superponibles. En la cohorte prospectiva, espironolactona fue suspendida en 9 pacientes (9,9%) por efectos adversos.
ConclusionesTras 3 meses con espironolactona se observó un descenso de PA asociado a descenso del FGe y aumento de Creap y Kp, cambios que se mantuvieron a los 12 meses. Espironolactona es un tratamiento eficaz y seguro para la HTAR en pacientes con FGe basal ≥30ml/min/1,73m2.
Resistant arterial hypertension (RHTN) is defined as a poorly controlled blood pressure (BP) that persist despite treatment with at least 3 different types of antihypertensive drugs, including a diuretic, correctly combined and at adequate doses. Insufficient control of BP is considered if BP is above 140 and/or 90mmHg of systolic blood pressure (SBP) and/or diastolic blood pressure (PAD), respectively, or values above 130 and/or 80mmHg obtained by of 24-h ambulatory blood pressure monitoring.1–3
Currently, RHTN is a major public health problem, since its prevalence is estimated to be 10–20% of the hypertensive population on treatment.2–4 As compared with patients with controlled BP those with RHTN have a higher risk of presenting major cardiovascular events.4,5
The new clinical practice guidelines of the European Society of Hypertension and the European Society of Cardiology recommend the use of spironolactone, an aldosterone receptor antagonist, as the fourth drug in patients with RHTN.3 In recent years, a number of studies have confirmed the antihypertensive efficacy of spironolactone6–9 in addition to its beneficial effect on myocardial fibrosis and ventricular remodeling,10 and proteinuria.11 For all these reasons, the prescription of spironolactone has been increasing in recent years. This increase in the use of spironolactone has been also stimulated by the publication of various studies evaluating therapeutic effects and the possible associated adverse effects, that are mainly related to increased plasma potassium (K) or worsening of kidney function. These studies have been focused on the indication of spironolactone for the reduction of proteinuria in patients with diabetes mellitus11,12 or ventricular dysfunction13,14 in patients with or without chronic kidney disease.15 Likewise, there are studies on the use of spironolactone in RHTN in which the main objective is antihypertensive efficacy.6–9 However, there are only few studies that report results not only in terms of antihypertensive efficacy but also in renal functional changes, both early and at medium-long term.
The objective of this study is to evaluate the risks and benefits of using spironolactone for the treatment of HTAR.
MethodsPopulationObservational study of 216 patients with RHTN with spironolactone added to the antihypertensive regimen (initial dose of 12.5–25mg per day), being followed in the Hypertension Unit of the Nephrology Service of the Hospital del Mar in Barcelona. The first 125 patients included were evaluated between June 2007 and March 2016, and were analyzed retrospectively (cohort I). Subsequently, the study was expanded with 91 additional patients of the same characteristics, who were prospectively analyzed (cohort II) from April 2016 to September 2018 (90 completed the study at 3 months, 85 at 6 months and 75 at 12 months).
The specific objectives were to evaluate: (a) the antihypertensive efficacy of spironolactone; (b) the change in plasma creatinine (Cr), and in the estimated glomerular filtration rate (eGFR) by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula, and the modification of plasma potassium (K) concentration. These parameters were evaluated at 3, 6 and 12 months after the initiation of the spironolactone.
Patients include were 18 years or older, diagnosed with RHTN according to the guidelines of the European Society of Hypertension3 as BP >140/90mmHg despite treatment with 3 or more antihypertensive drugs of different therapeutic classes including a diuretic. In most cases the diagnosis of RHTN was confirmed by 24-hour ambulatory blood pressure monitoring (ABPM).3 Exclusion criteria were chronic kidney disease grades 4, 5 or 5D, and a basal plasma K >5mmol/l.
Variables studiedClinical and laboratory data were evaluated at baseline and at 3, 6, and 12 months after starting spironolactone. In addition, in cohort II those cases in whom the spironolactone was discontinued by the physician were evaluated and the reason for discontinuation of the medication were analyzed. Demographic information and data from medical records were collected, including age, sex, smoking, weight and body mass index (BMI), cardiovascular disease (ischemic heart disease, heart failure, cerebrovascular disease and peripheral vascular disease), diabetes mellitus, dyslipidemia, kidney disease, obstructive sleep apnea syndrome (OSA), measurements of BP in the outpatient clinic and 24-h ABPM. Laboratory parameters (Cr, eGFR, K concentration, and urine albumin/creatinine ratio) were obtained at baseline. During the follow-up, the BP measurement and laboratory parameters were obtained in the outpatient clinic at 3, 6 and 12 months after starting spironolactone. In addition, at 12 months, BP was also measured by 24-h ABPM in 108 patients.
The eGFR was estimated using the CKD-EPI equation.16 Albuminuria was measured in the first urine sample in the morning using immunonephelometric method and it is expressed as the albumin/creatinine ratio. Biochemical parameters were analyzed by autoanalyzer using standard methods.
The BP was measured using a validated semi-automatic device (Omron 705IT) with a cuff of adequate size for the brachial perimeter of each subject. In each visit, the BP was measured after 5min resting with the patient sitting, BP was measured 3 times with a 1–2min interval and the average of the 3 measurements was considered the definitive value.
Prescribed antihypertensive drugs at baseline and 12 months after starting spironolactone were recorded. Adherence to treatment was evaluated from the information self-reported by the patient, with systematic review of the treatment in each visit.
Statistical analysisCentral tendency statistics, arithmetic mean (95% confidence interval) were used for continuous variables and frequency distribution for discrete variables. Other central tendency statistics, median (25th and 75th percentiles) were used for continuous variables that did not follow a normal distribution. The evolution of the parameters of interest throughout the follow-up was evaluated by mixed linear models. Changes in albuminuria, after 12 months of spironolactone were evaluated by Wilcoxon test since values of albuminuria are not normally distributed.
All analysis were adjusted for the variables age, sex, BMI, diabetes mellitus, OSA, and eGFR. p<0.05 values were considered statistically significant. The STATA 15.1 program (StataCorp, CollegeStation, TX, USA) was used for statistical analysis.
ResultsThere were a total of 216 patients included with a mean age of 66 years, most of them Caucasians men, with a high prevalence of diabetes mellitus, dyslipidemia, and OSA (Table 1).
Baseline characteristics of patients with resistant arterial hypertension (RHTN).
| RHTN (n=216) | |
|---|---|
| Age, years (mean±SD) | 65.6±11.6 |
| Male sex, n (%) | 136 (63) |
| Caucasian race, n (%) | 199 (92.1) |
| BMI, kg/m2 (mean±SD) | 31.9 (5.2) |
| Abdominal circumference, cm (mean±SD) | 112.7±13.2 |
| Type 2 diabetes mellitus, n (%) | 95 (44) |
| Dyslipidemia, n (%) | 161 (74.5) |
| eGFR, ml/min/1.73m2 (mean±SD) | 76.9±21.2 |
| CKD stage 3, n (%) | 45 (20.8) |
| Albuminuria, mg/g, median (RIQ25–75) | 18.8 (6.3–83.8) |
| Micro/macroalbuminuria, n (%) | 60 (27.8) |
| CKD stage 3 and micro/macroalbuminuria, n (%) | 18 (8.3) |
| OSA, n (%) | 62 (28.7) |
| Ischemic heart disease, n (%) | 27 (12.5) |
| Cerebrovascular disease, n (%) | 15 (6.9) |
| Peripheral vascular disease, n (%) | 16 (7.4) |
SD: standard deviation; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; RHTN: resistant arterial hypertension; BMI: body mass index; RIQ: interquartile range; OSA: obstructive sleep apnea syndrome.
Before starting spironolactone, patients were on an average of 4±1 antihypertensive drugs: 100% diuretics (74.2% thiazide and 25.8% loop diuretic), 93.5% a renin-angiotensin-aldosterone system blocker (79.1% ARA-II and 14.4% ACEI), 87.5% calcium antagonist, 59.7% a beta-blocker, 38.0% an alpha-blocker, 10.2% a sympatholytic (clonidine or moxonidine), 3, 2% a direct renin inhibitor (aliskiren) and 1% an arterial vasodilator (hydralazine).
The initial daily dose of spironolactone (median, interquartile range (IQR 25–75)) was 25mg (12.5–25) in cohort I and 12.5mg (12.5–25) in cohort II, and at 12 months t was 25mg (25–50) and 25mg (12.5–25) in cohorts I and II, respectively.
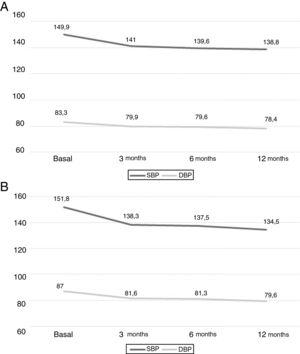
EfficiencyThe BP decreased significantly at 3 months of treatment (p<0.001) and this reduction was maintained at 12 months. The initial BP, mean (95% CI) was 149.9mmHg (146.7–153.1) and 151.8mmHg (148.1–155.5) in cohorts I and II respectively. The final BP was of 138.8mmHg (135.2–142.3) and 134.5mmHg (130, 2–138.9) for cohorts I and II, respectively. No significant changes were observed between 3 and 6 months, between 6 and 12 months, and between 3 and 12 months (p>0.05 for all comparisons). Fig. 1 shows the results of both cohorts.
In addition, changes in BP obtained with 24-h ABPM in108 patients at baseline and after 12 months of spironolactone treatment are shown in Table 2. At 12 months, the 24-hour SBP decreased −13.9mmHg (−18.4 to −9.4) and the 24-h DBP was also reduced −6.1mmHg (−9.3 to −2.9), p<0.001 in both cases. A 46.3% of patients changed their initial status from uncontrolled RHTN to controlled BP which means a 24-h SBP ≤130mmHg and DBP ≤80mmHg after 12 months of treatment with spironolactone. These changes were not related to changes in weight, −0.17kg (−1.2 to +0.5) p=0.5, or BMI, −0.14kg/m2 (−0.6 to +0.2) p=0.7.
Change in blood pressure by 24h – ABPM – during treatment with spironolactone.
| BP | Cohort I | Cohort II | ||||
|---|---|---|---|---|---|---|
| Basal (mmHg) | 12 months (mmHg) | p | Basal (mmHg) | 12 months (mmHg) | p | |
| SBP 24h | 147.0 | 134.7 | <0.001 | 146.7 | 126.3 | <0.001 |
| DBP 24h | 78.0 | 74.4 | <0.001 | 81.3 | 74.4 | 0.008 |
| Daytime SBP | 149.7 | 137.9 | <0.001 | 149.2 | 128.1 | <0.001 |
| Daytime DBP | 80.0 | 74.5 | <0.001 | 83.6 | 74.4 | 0.005 |
| Night SBP | 140.2 | 127.3 | <0.001 | 140.4 | 125.7 | 0.005 |
| Night DBP | 71.2 | 67.1 | 0.02 | 75.3 | 67.8 | 0.008 |
ABPM: ambulatory blood pressure monitoring; BP: blood pressure; DBP: diastolic blood pressure; SBP: systolic blood pressure.
Considering the circadian BP patterns, 62.1% of the patients initially presented a non- dipper or riser pattern and after 12 months on spironolactone this proportion decreased to 48.9% (p=0.001). A 20.9% of the subjects presented a favorable change in the circadian pattern with respect to the initial ABPM (going from riser to non-dipper, from riser to dipper or from non-dipper to dipper) (p=0.012).
Urinary albumin/creatinine decreased from an initial median (IQ range 25–75) of 18.8mg/g (6.3–83.8) to 9.5mg/g (3.9–31.0) at 12 months, p<0.001. This decrease was especially relevant in patients with micro/macroalbuminuria in whom the initial and final urinary albumin excretion (median [IQ range 25–75]) was 149.7mg/g (58.3–447.9) and 44 0.0mg/g (13.0–154.8) respectively, p<0.001.
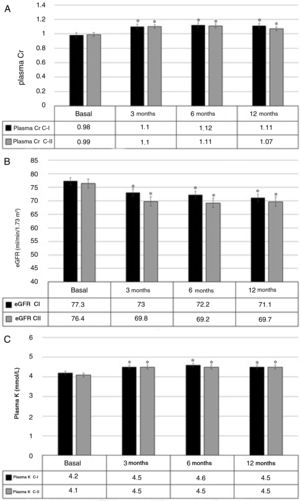
SafetyThe plasma Cr increased by 0.10mg/dL (0.07–0.14), p<0.001 after 3 months. This increase remained stable at 6 and 12 months, without significant additional change in these both periods. Fig. 2 A shows the mean values of plasma Cr in cohorts I and II separately.
The initial eGFR was 76.9ml/min/1.73m2 (range 72.7–81.0). At 3 months, there was a decrease of −5.4ml/min/1.73m2 (−7.3 to −3.5), p<0.001. The reduction in the eGFR remained stable at 6 and 12 months, with no significant differences between these two periods; Fig. 2B, shows the values of the two cohorts.
At 3 months the plasma K increased by 0.3mmol/l (0.2–0.4) (p<0.001). This increase remained stable without significant differences at 6 and 12 months. Fig. 2C shows the results from both cohorts separately.
In cohort II, spironolactone was withdrawn in 9 of the 91 patients (9.9%). The causes were: gastrointestinal intolerance (one patient at 6 months and 3 patients at 12 months), decrease in eGFR>20% (2 patients after 3 months of treatment), gynecomastia (one patient), dysmenorrhea (one patient) and hyperkalemia (one patient >5.5mEq/l at 12 months).
DiscussionThe present study confirms that the use of spironolactone to treat RHTN produces a significant decrease in BP that is evident at 3 months, and it is maintained at 12 months. This decrease is observed not only in the BP values obtained in the out patient clinic, but also in 24-h ABPM. The adverse effects such as the decrease in the eGFR and the increase in the plasma K, are present after 3 months of starting spironolactone and remained stable thereafter.
Spironolactone is a mineralocorticoid receptor antagonist with a potent antihypertensive effect. Our study showed tha spironolactone produced a reduction of BP of 10.9 and 4.3mmHg in SBP and DBP respectively, results that are similar to previous studies.6,7 Likewise, the reduction in BP observed by 24-h ABPM is also comparable to the published data.7–9 Our study also provides information on the changes in ABPM pattern after 12 months on spironolactone, highlighting a significant reduction of the percentage of patients with an initial no-dipper or riser pattern implying that there was an improvement in the cardiovascular risk of these patients. Regarding the antiproteinuric effect of spironolactone, our study shows a significant reduction in albuminuria of 50% (around 9.3mg/g). Van den Meiracker et al.11 found similar antiproteinuric effect in type 2 diabetes mellitus with higher dose of spironolactone, 25–50mg/day, but there were no significant differences observed between the placebo and spironolactone groups. Bianchi et al.,12 added 25mg/day of spironolactone to the conventional antihypertensive treatment and observed a reduction in proteinuria but did not observe differences between placebo and spironolactone.
The use of spironolactone is limited by the concern of adverse effects, especially a deterioration of kidney function. These adverse effects have been analyzed mainly in studies where spironolactone was used for treatment of proteinuria in diabetic patients11 or ventricular dysfunction in patients with heart failure.13,14 In the aforementioned studies it was observed a progressive deterioration of renal function, that was accelerated during the first 3 months and more gradual but progressive at long term. In relation to plasma K concentration, we have observed an initial increase after the initiation of spironolactone, which stabilizes thereafter. Regarding the use of spironolactone in RHTN, the PRAGUE-158 study did not show significant variations in eGFR or plasma K after 12 months of treatment with spironolactone, but only 19 patients completed 12 months of spironolactone treatment. By contrast, the PATHWAY-2 study6 showed an increase in plasma K (0.45mmol/l) after starting spironolactone, and a decrease in the eGFR was observed in all groups in which an antihypertensive agent (spironolactone, bisoprolol or doxazosin) was added. Withdrawal of antihypertensive treatment for worsening kidney function and hyperkalemia was not significantly different between the various treatment groups. The changes in renal function were evaluated 6 months9 after starting treatment; our study allows a longer period of observation, up to 12 months.
The antihypertensive efficacy of spironolactone is evident as early as 3 months, for both SBP and DBP, and the BP remains stable, without significant changes, at 6 and 12 months. Likewise, the adverse effects regarding the decrease of eGFR and the increase in plasma K are observed 3 months after the initiation of spironolactone and, subsequently, they remain stable at 6 and 12 months. The mean spironolactone dose was not reduced throughout treatment.
The prospective follow-up cohort of the present study shows that basically in one out of 10 patients the spironolactone is discontinued, and the main cause is gastrointestinal intolerance. Renal adverse effects forced discontinuation of treatment in 3 of 91 patients (3.3%), an acceptable rate considering the overall risk/benefit balance shown in this analysis.
The effects of spironolactone in terms of efficacy and adverse effects, are evident 3 months after the introduction of the drug, possibly in relation to hemodynamic changes that take place as an adaptive response to the effects of the drug according to its mechanism of action. Overall, these data suggest that there is an adaptation to these hemodynamic changes, with stabilization of both the decrease in BP and the adverse effects.
The main limitations of the study is the fact that it is an observational study, although this is partially offset by the cohort of patients followed prospectively. Another limitation is that the study is performed in a single center and clinical practice is very homogeneous for all patients. The exclusion of patients with chronic kidney disease grades 4, 5 or on dialysis, prevents drawing conclusions on these populations.
In conclusion, spironolactone is a safe and effective drug in the treatment of RHTN in patients with a baseline eGFR ≥30ml/min/1.73m2.
FinancingThis study has received partial financing from the ISCIII project – RETICS Subprogram, FEDER funds and REDinREN RD16/0009/0013.
Conflicts of interestThe authors declare that they have no conflicts of interest related to the content of this article.
Please cite this article as: Galceran I, Vázquez S, Durán X, Outón S, Pascual J, Oliveras A. Seguridad renal de espironolactona en pacientes con hipertensión arterial resistente. Nefrologia. 2020;40:413–419.