Steroid withdrawal in renal transplantation is desirable to avoid their adverse effects. However, by decreasing the immunosuppression, could lead to an increased risk for the development of HLA-Abs.
ObjectiveEvaluate the relationship between steroid withdrawal and development of HLA-Abs in renal transplantation.
MethodsWe analyzed sera by Luminex from 182 kidney transplants performed from 1998 to 2011, before and two years after transplantation. All the patients had a pretransplant PRA (panel reactive of antibodies) <20% by complement-dependent cytotoxicity (CDC) and maintenance immunosuppression with tacrolimus and mycophenolate mofetil (MMF). We compared a group of steroid withdrawal at 7 months (group-I; n=130) and another control with non-withdrawal (group-II; n=52).
Results22 patients (16.9%) in group-I and 11 patients in group-II (21.1%) had HLA-Abs after two years (pNS). Despite excluding patients with PRA >20%, we detected HLA-Abs pretransplant by Luminex in 11.5% of patients in both groups, of which, 66.6%, versus 53% (p 0.058), developed new specificities, with a similar percentage of donor specific antibodies (DSA) in both groups (33.33% vs 36.36%), pNS. In the subgroup without pretransplant HLA-Abs (group-I; n=115, group-II; n=45), 6.08% developed de novo HLA-Abs, being DSA 3.4% (Group-I) versus 7.69% in group II with 3.84% DSA (pNS).
ConclusionsSteroid withdrawal at 7 months of renal transplantation does not entail a higher risk in terms of HLA-Abs development in patients without pretransplant HLA-Abs and treatment with tacrolimus and MMF, although larger studies are needed to confirm these findings.
La retirada de esteroides en el trasplante renal es deseable por sus efectos adversos, sin embargo, al disminuir la inmunosupresión podría conllevar un riesgo superior para el desarrollo de Ac-anti-HLA.
ObjetivoEvaluar la relación entre la retirada de esteroides y el desarrollo de Ac-anti-HLA en el trasplante renal.
MétodosSe evaluaron los sueros por Luminex de 182 trasplantados renales desde 1998 a 2011, antes y a los 2 años del trasplante. Todos tenían un panel reactivo frente a anticuerpos (PRA)<20% pretrasplante por citotoxicidad dependiente de complemento y mantuvieron la inmunosupresión con tacrolimús y micofenolato mofetilo (MMF). Comparamos un grupo de retirada de esteroides a los 7 meses (grupo i; n=130) y otro de no retirada (grupo ii; n=52).
Resultados22 pacientes (16,9%) en el grupo i y 11 pacientes en el grupo ii (21,1%) presentaban Ac-anti-HLA a los 2 años (pNS). A pesar de excluir a los pacientes con PRA>20%, detectamos Ac-anti-HLA pretrasplante por Luminex en el 11,5% de los pacientes en ambos grupos, de los cuale, desarrollaron nuevas especificidades el 66,6% del grupo i y el 53% en el grupo ii (p 0,058), con un similar porcentaje de anticuerpos donante específicos (DSA) (33,3% vs. 36,36%), pNS. En el subgrupo sin Ac-anti-HLA pretrasplante (grupo i; n=115; grupo ii; n=45), el 6,08% desarrollaron Ac-anti-HLA de novo, siendo DSA el 3,4% (grupo-I) vs. 7,69% con DSA en el 3,84% (grupo-II), pNS.
ConclusionesLa retirada de esteroides a los 7 meses del trasplante renal no conlleva un riesgo superior en términos de desarrollo de Ac-anti-HLA en aquellos pacientes sin anticuerpos pretrasplante y en tratamiento con tacrolimús y MMF, aunque se requieren estudios más amplios para confirmar estos hallazgos.
The detection of antibodies against the HLA system (HLA-Abs) in transplantation has been linked to the development of acute and chronic antibody mediated rejection (ABMR)1 in clinical and subclinical forms of presentation, and remains as a major cause of graft losses.2 Monitoring HLA-Abs has become a routine practice in the clinical follow up of renal transplant recipients3 that helps to indicate a graft biopsy and early treatment.
The development of de novo HLA-Abs is usually associated with modifications in the immunosuppressive treatment. In clinical practice, the modulation of the immunosuppressive regimen is common in order to avoid the long-term adverse effects. The calcineurin inhibitors (CNI) withdrawal or conversion to mTOR-inhibitor is currently performed to reduce nephrotoxicity or risk of malignancy.4,5 These changes in immunosuppression are risk of development of new HLA-Abs and consequently ABMR.6,7
The steroids withdrawal is also desirable to avoid their adverse effects on long-term treatment, but its relationship with the development of new HLA-Abs is not yet well established.
One of the most important adverse effects of steroids is increased cardiovascular risk. The steroid maintenance has been linked to the onset of diabetes mellitus, dyslipidemia, hypertension and obesity.8,9 In a study involving 68,781 patients who received prednisone versus 82,200 patients who did not, it was observed that the patients who received doses of prednisone ≥7.5mg/day had a relative risk of 2.5 for cardiovascular events.10 In addition, the rate of death with a functioning graft is high (40%) being cardiovascular events the most frequent cause.11 Therefore, the maintenance of steroids could be a modifiable cause of death with a functioning graft.
Currently, there are potent immunosuppressants in maintenance therapy (Tacrolimus and Mycophenolic mofetil-MMF) and the steroids withdrawal seems plausible, at least in those patients with low immunological risk. Steroids withdrawal has risk of acute rejection and higher slope of glomerular filtration rate (GFR) loss in the long term. We hypothesize that steroids elimination can contribute to production of donor specific antibodies (DSA). So, the aim of our study is to assess the relationship between steroid withdrawal and development of HLA-Abs after renal transplantation.
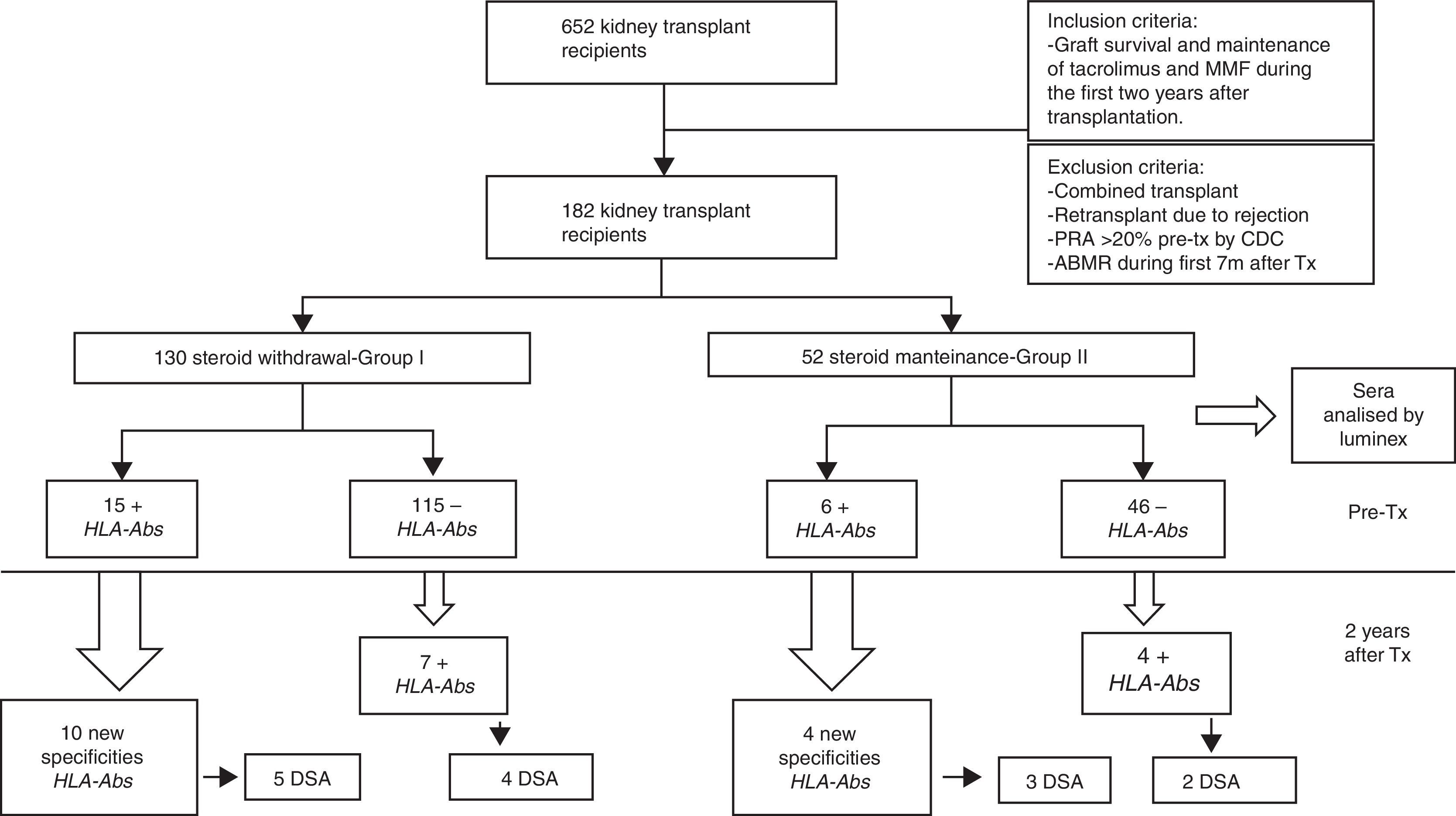
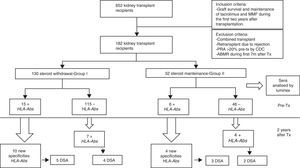
Material and methodsPatientsWe studied 652 kidney transplant recipients, performed in University Hospital Marqués de Valdecilla, Santander (Spain) from January 1998 to December 2011 who met the following criteria: graft survival of at least two years and maintenance of immunosuppression with tacrolimus and MMF during the first two years after transplantation. We excluded of the study: combined transplants; retransplants which lost the first transplant for rejection; panel reactive of antibodies (PRA) pretransplant >20% by complement-dependent cytotoxicity (CDC), acute antibody-mediated rejection (ABMR) during the 7 months after transplantation. Finally, 182 kidney transplant recipients fulfilled the inclusion criteria and were included in the study.
The patient data were obtained from the prospectively maintained database of renal transplant patients at our centre. The demographic, immunologic and clinical parameters are summarized in Table 1. Patients were classified into two groups according to steroid withdrawal: Group-I: removal of steroids at 7 months posttransplant (n=130) and Group-II: steroids maintenance during the first two years after transplantation (n=52).
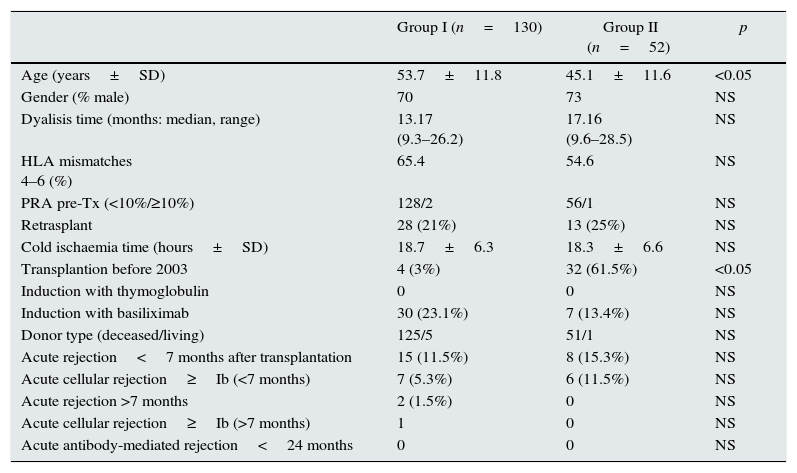
Demographic, immunologic and clinical parameters of the patients included in the study (Group I: steroid withdrawal; Group II: non steroid withdrawal).
| Group I (n=130) | Group II (n=52) | p | |
|---|---|---|---|
| Age (years±SD) | 53.7±11.8 | 45.1±11.6 | <0.05 |
| Gender (% male) | 70 | 73 | NS |
| Dyalisis time (months: median, range) | 13.17 (9.3–26.2) | 17.16 (9.6–28.5) | NS |
| HLA mismatches 4–6 (%) | 65.4 | 54.6 | NS |
| PRA pre-Tx (<10%/≥10%) | 128/2 | 56/1 | NS |
| Retrasplant | 28 (21%) | 13 (25%) | NS |
| Cold ischaemia time (hours±SD) | 18.7±6.3 | 18.3±6.6 | NS |
| Transplantion before 2003 | 4 (3%) | 32 (61.5%) | <0.05 |
| Induction with thymoglobulin | 0 | 0 | NS |
| Induction with basiliximab | 30 (23.1%) | 7 (13.4%) | NS |
| Donor type (deceased/living) | 125/5 | 51/1 | NS |
| Acute rejection<7 months after transplantation | 15 (11.5%) | 8 (15.3%) | NS |
| Acute cellular rejection≥Ib (<7 months) | 7 (5.3%) | 6 (11.5%) | NS |
| Acute rejection >7 months | 2 (1.5%) | 0 | NS |
| Acute cellular rejection≥Ib (>7 months) | 1 | 0 | NS |
| Acute antibody-mediated rejection<24 months | 0 | 0 | NS |
Both groups were comparable in terms of gender, time on dialysis, retransplants, cold ischaemia time, HLA mismatches, pretransplant sensitization (tested by CDC), induction therapy with thymoglobulin or basiliximab, and acute rejection during the 7 months after transplantation. The Group II patients were significantly younger and predominate transplants before 2003, as the only statistically significant differences. The mean study follow-up was two years after transplantation.
Steroid withdrawal protocolIn 2003, we started a protocol with steroid withdrawal up to 7 months after kidney transplantation in patients treated with tacrolimus and MMF, and excluding those patients with high immunological risk (acute ABMR; acute cellular rejection with a degree>Ib of the Banff classification in the 7 months after transplantation, pretransplant PRA>20% or rejection with graft loss in prior transplants). However, even before 2003, in some patients, steroids were withdrawn at 7 months with the same criteria.
An earlier withdrawal was not performed at our centre because we do not use induction thymoglobuline routinely, and decided to make a slow pattern of steroids that ended at 7 months.
The prednisone dose reduction consisted in gradually decreasing the dose until the complete withdrawal up to 7 months after transplantation: 15mg/d at 15 days; 10mg/d a day at one month; 7.5mg/d at two months; 5mg/d at three months; 5mg/d and 2.5mg/d alternating at four months; 2.5mg/d at five months; 2.5mg every other day at six months until complete withdrawal at seven months.
Serum samples selection and sera analysisSerum samples of all transplanted patients were routinely collected and stored at −80°C. Frozen serum samples were selected to investigate the presence of HLA-Abs. For every patient, two samples were selected, one immediately before transplantation and another one two years after transplantation. The sera were analyzed by LABScreen®Mixed (One Lambda Inc., Canoga Park, CA, USA) for screening antibodies and LABScreen®Single antiHLA Antigen (One Lambda) class-I and/or class-II if were positive in the screening. A total of 372 serum samples were analyzed.
A mean fluorescence intensity (MFI) value greater than 1500 was considered positive for a particular HLA specificity.
Statistical methodsQuantitative variables were compared using the t-Student's test. The percentages were obtained with the chi-square test. Differences were considered significant when p-value was below 0.05.
ResultsTwenty-two patients (16.92%) in group I and eleven patients (21.15%), in Group II (pNS) presented serum HLA-Abs at two years after transplantation (Table 2).
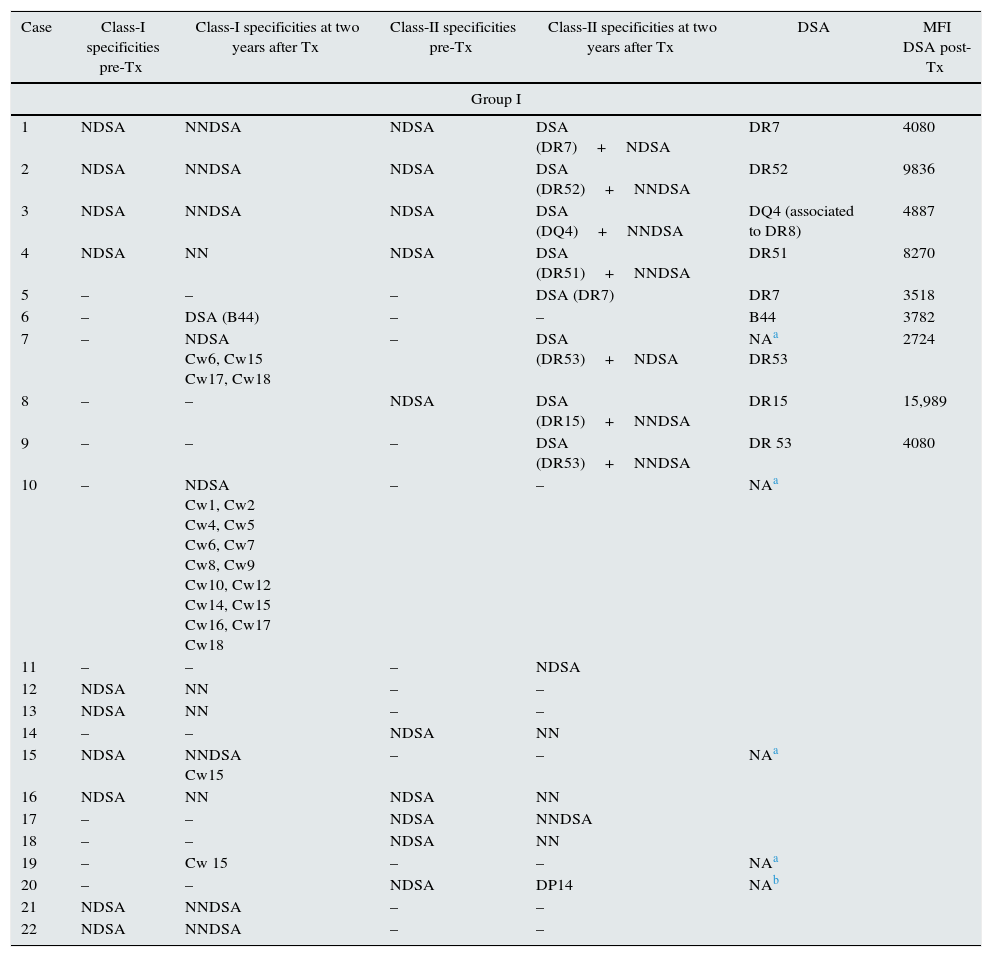
List of specificities HLA-Abs detected pretransplant and posttransplant. DSA with the MFI value.
| Case | Class-I specificities pre-Tx | Class-I specificities at two years after Tx | Class-II specificities pre-Tx | Class-II specificities at two years after Tx | DSA | MFI DSA post-Tx |
|---|---|---|---|---|---|---|
| Group I | ||||||
| 1 | NDSA | NNDSA | NDSA | DSA (DR7)+NDSA | DR7 | 4080 |
| 2 | NDSA | NNDSA | NDSA | DSA (DR52)+NNDSA | DR52 | 9836 |
| 3 | NDSA | NNDSA | NDSA | DSA (DQ4)+NNDSA | DQ4 (associated to DR8) | 4887 |
| 4 | NDSA | NN | NDSA | DSA (DR51)+NNDSA | DR51 | 8270 |
| 5 | – | – | – | DSA (DR7) | DR7 | 3518 |
| 6 | – | DSA (B44) | – | – | B44 | 3782 |
| 7 | – | NDSA Cw6, Cw15 Cw17, Cw18 | – | DSA (DR53)+NDSA | NAa DR53 | 2724 |
| 8 | – | – | NDSA | DSA (DR15)+NNDSA | DR15 | 15,989 |
| 9 | – | – | – | DSA (DR53)+NNDSA | DR 53 | 4080 |
| 10 | – | NDSA Cw1, Cw2 Cw4, Cw5 Cw6, Cw7 Cw8, Cw9 Cw10, Cw12 Cw14, Cw15 Cw16, Cw17 Cw18 | – | – | NAa | |
| 11 | – | – | – | NDSA | ||
| 12 | NDSA | NN | – | – | ||
| 13 | NDSA | NN | – | – | ||
| 14 | – | – | NDSA | NN | ||
| 15 | NDSA | NNDSA Cw15 | – | – | NAa | |
| 16 | NDSA | NN | NDSA | NN | ||
| 17 | – | – | NDSA | NNDSA | ||
| 18 | – | – | NDSA | NN | ||
| 19 | – | Cw 15 | – | – | NAa | |
| 20 | – | – | NDSA | DP14 | NAb | |
| 21 | NDSA | NNDSA | – | – | ||
| 22 | NDSA | NNDSA | – | – | ||
| Group II | ||||||
|---|---|---|---|---|---|---|
| 1 | NDSA | – | NDSA | DSA (DR53)+NNDSA | DR53 | 11,371 |
| 2 | NDSA | NN | NDSA | DSA (DR8)+NNDSA | DR8 | 2368 |
| 3 | – | DSA (A2, A29)+NDSA | – | NDSA | A2 | 3567 |
| A29 | 2497 | |||||
| 4 | NDSA | NN | NDSA | DSA (DR4) +NNDSA | DR4 | 4613 |
| 5 | NDSA | NN | NDSA | NN | – | |
| 6 | NDSA | NN | – | – | ||
| 7 | NDSA | NNDSA | NDSA DP1, DP20, DP23, DPA1*02:01 DPA1*04:01 | DP13 | NAb | |
| 8 | – | – | NDSA | NN | ||
| 9 | – | – | – | NDSA | ||
| 10 | – | – | NDSA | NN | ||
| 11 | – | Cw4 | – | – | NAa | |
DSA: donor specific antibodies; NDSA: not donor specific antibodies; NNDSA: new not donor specific antibodies; NN: not new specificities; MFI: mean fluorescence intensity; Tx: transplantation.
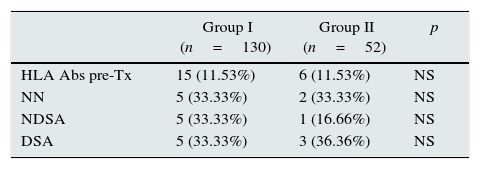
When pretransplant sera were analyzed by Luminex a total of fifteen patients (11.53%) in steroid withdrawal group, and six patients (11.53%) in the group of steroid maintenance presented HLA-Abs prior kidney transplantation, despite the exclusion criteria of PRA>20% by CDC. Of these patients, ten developed new specificities after two years (66.66%) from the group I, versus four patients (53.02%) from the group II (p 0.058), with a similar percentage of DSA (33.33% in group I vs 36.36% in group II) (Table 3).
Patients with HLA-Abs pretransplant, and differentiation according to the specificities observed after two years of transplantation.
| Group I (n=130) | Group II (n=52) | p | |
|---|---|---|---|
| HLA Abs pre-Tx | 15 (11.53%) | 6 (11.53%) | NS |
| NN | 5 (33.33%) | 2 (33.33%) | NS |
| NDSA | 5 (33.33%) | 1 (16.66%) | NS |
| DSA | 5 (33.33%) | 3 (36.36%) | NS |
NN: not new specificities; NDSA: not donor specific antibodies; DSA: donor specific antibodies.
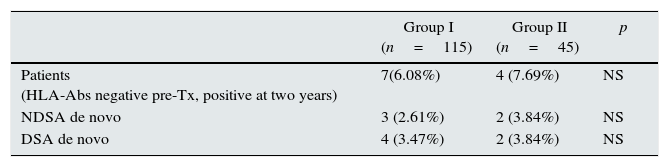
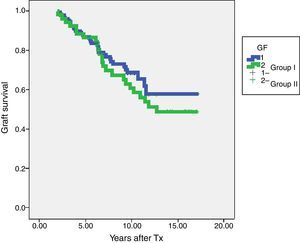
Within the patients without HLA-Abs before kidney transplantation (group I-n=115; group II-n=45), seven patients in steroid withdrawal group (6.08%) developed de novo HLA-Abs, four of them being DSA (3.47%) whereas four patients in the group II (7.69%) developed de novo HLA-Abs and two of them were DSA (3.84%); pNS (Table 4). Therefore, in the subgroup without preformed antibodies, there are not differences in the development of antibodies in both groups (6.08% vs 7.69%, pNS), although steroid withdrawal (Fig. 1).
Patients without HLA-Abs before transpantation and the HLA-Abs at two years after transplantion.
| Group I (n=115) | Group II (n=45) | p | |
|---|---|---|---|
| Patients (HLA-Abs negative pre-Tx, positive at two years) | 7(6.08%) | 4 (7.69%) | NS |
| NDSA de novo | 3 (2.61%) | 2 (3.84%) | NS |
| DSA de novo | 4 (3.47%) | 2 (3.84%) | NS |
NDSA: not donor specific antibodies; DSA: donor specific antibodies; De novo: specificities with new development; Tx: transplantation.
Only two patients (1.5%) in group I had acute cellular rejection after steroid withdrawal, with grades Ia and Ib of Banff classification. The last one had already pretransplant HLA-Abs, and developing a DSA (DQ4) at two years. The other did not develop HLA-Abs. None of recipients in both groups had ABMR during the study period.
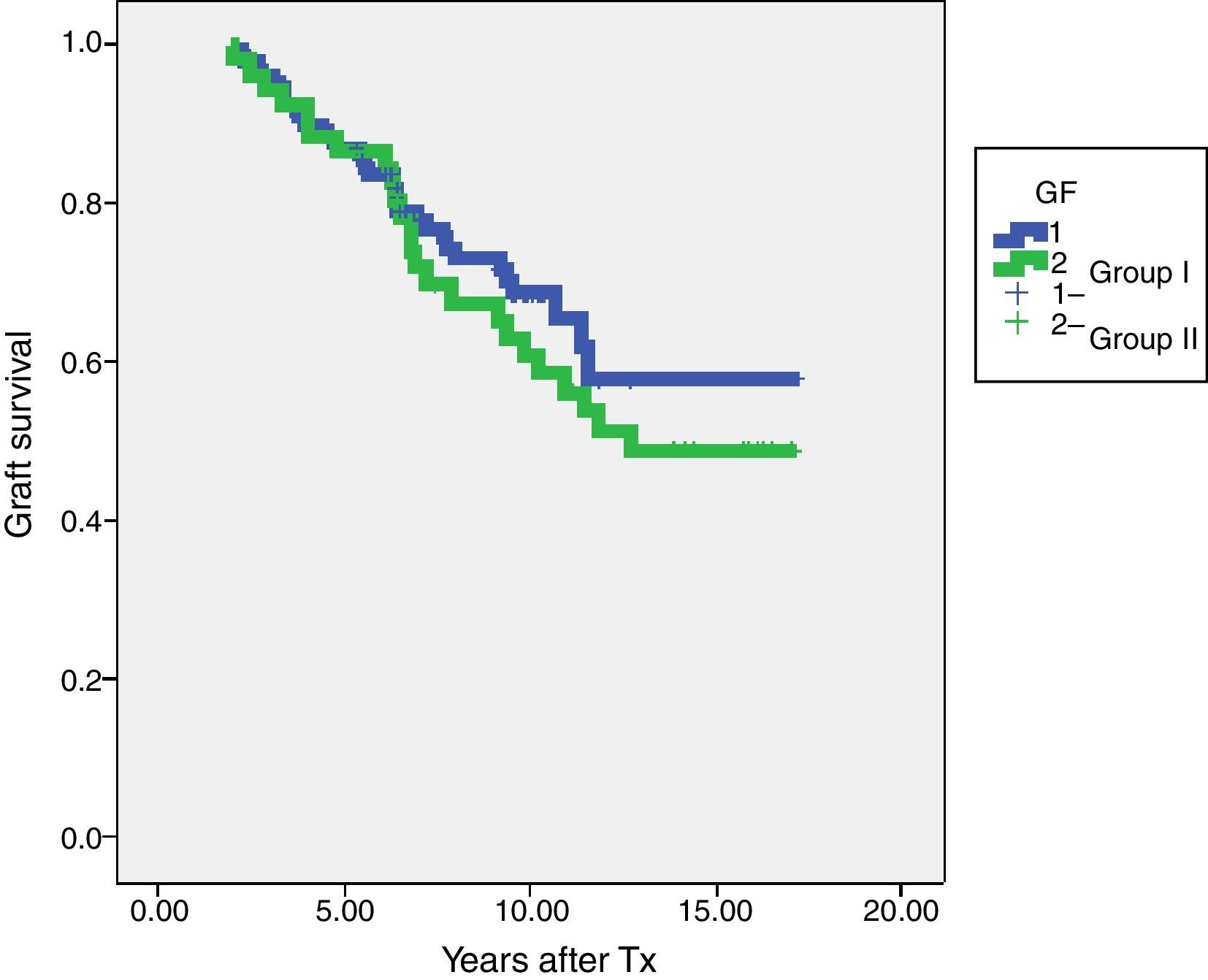
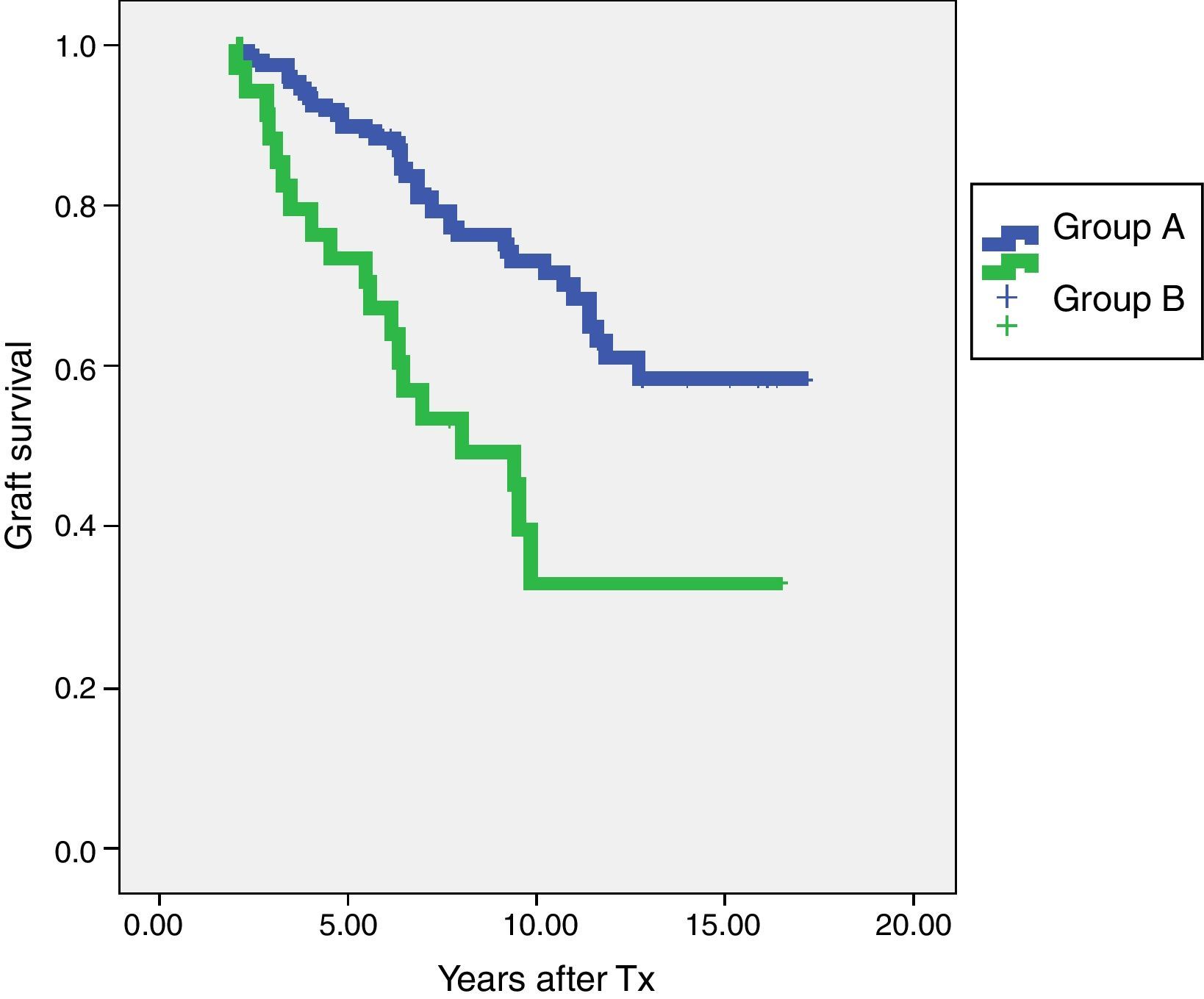
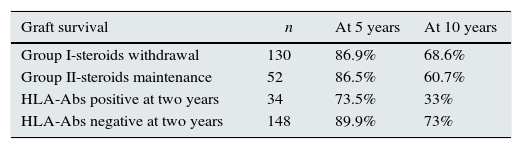
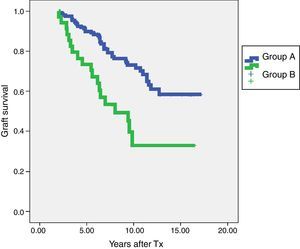
In terms of graft survival, no statistically significant differences in both groups (Fig. 2) are appreciated, however those patients with HLA-Abs at two years after transplantation have worse survival (Fig. 3 and Table 5). The influence of HLA Abs is confirmed in graft survival, after multivariate analysis (p<0.001 and HR 2.758, 95% CI 1597–4764).
Graft survival according to steroids withdrawal/maintenance and presence of HLA-Abs at two years after transplantation.
| Graft survival | n | At 5 years | At 10 years |
|---|---|---|---|
| Group I-steroids withdrawal | 130 | 86.9% | 68.6% |
| Group II-steroids maintenance | 52 | 86.5% | 60.7% |
| HLA-Abs positive at two years | 34 | 73.5% | 33% |
| HLA-Abs negative at two years | 148 | 89.9% | 73% |
The minimization of immunosuppression could increase the risk of HLA-Abs development as previously demonstrated in CNI conversion to imTOR.6,7 Steroids withdrawal are not been studied in relation to the development of DSA in organ transplantation. The huge advantages of steroids elimination in relation to metabolic and cardiovascular adverse events may not be sufficient if a significant immunological risk is demonstrated.
There is only one study that analyzes the production of HLA-Abs in kidney transplants in adults, after early steroid withdrawal (7 days post-transplant). They concluded that steroid withdrawal at 7 days posttransplant was not at higher risk for developing HLA antibodies, but all patients have received induction with thymoglobuline.12 Our study is the first to assess the relationship between intermediate steroid withdrawal and the detection of serum HLA-Abs in renal transplants with standard immunosuppression (tacrolimus and MMF).
Our data suggest that steroid withdrawal up to 7 months after transplantation does not imply a higher risk of HLA-Abs development in patients with low immunological risk, and, more specifically, in patients who do not have HLA-Abs before transplantation. On the contrary, in patients with preformed HLA-Abs, the frequency of new HLA-Abs after steroid withdrawal increased (66.66%) as compared with non-withdrawal group (53.02%; p 0.058). Although there is no statistically significant difference, a higher tendency is observed in the group of steroid withdrawal, but further study is needed to confirm this point. This is in agreement with previous results from our group in kidney recipients converted from CNI therapy to mTORi therapy.7
The primary goal of the study was to observe the development of HLA-Abs after steroid withdrawal, not to assess the effect on rejection or graft survival. However, we have analyzed graft survival, detecting worse survival in those with anti-HLA antibodies. We have found no differences between the Group I and II. In the study period, only two patients in group I (1.5%) had a acute cellular rejection after steroid withdrawal. There are well-designed studies addressing this point. In a meta-analysis, Knight et al.13 observed that the acute rejection rate was slightly higher in the group of steroid withdrawal, but most of it of low-grade (class I-Banff classification). This group also showed a tendency to increase the rate of graft loss censored death. This trend disappears when all graft losses are included, including death with a functioning graft. Such data probably reflect the benefit on patient survival by removing the steroids to reduce cardiovascular risk. The studies included in the meta-analysis presented different moments of withdrawal, from the first day after transplantation up to 24 months after transplantation. In contrast, Opelz et al., 14 in a prospective study following 7 years after transplantation, compared a group with steroid withdrawal not earlier than 6 months after transplantation (1100 patients), and a control group with steroids maintenance (3045 patients). They observed benefits in the group that had undergone steroid withdrawal in terms of graft survival, patient survival, graft survival censoring death, and improvement in cardiovascular risk factors. They detected no difference in the rate of acute rejection or dysfunction renal in both groups, unlike other studies (with fewer patients) in which the steroid withdrawal was earlier and more acute rejection rate.15
Currently, there is no universal steroid withdrawal protocol. In accordance with the study of Opelz et al., it seems safe enough to remove steroids after 6–7 months of transplantation, as we followed in our protocol.
The present study has a number of limitations. First of all, it is an observational study. Although the steroid withdrawal protocol at 7 months after transplantation in patients with low immunological risk was established in 2003, it was not universally applied to all patients. Nonetheless, between 1998 and 2003, some patients had a steroid withdrawal at 7 months at our centre. This has allowed us to have a time-matched group in steroids maintenance. The withdrawal protocol was followed by patients without acute rejection ≥ Ib of Banff classification, however a small percentage of patients (5.3%) with such rejection grades were removed from steroids at 7 months, only one of them developed DSA, but he had preformed antibodies, and the remaining patients did not develop antibodies. In the group with steroids maintenance, the frequency of patients with acute cellular rejection ≥ Ib was higher (11.5%), and this is one reason why steroids were kept. None of these patients developed antibodies at two years.
We also observed that patients from the steroids withdrawal group were older. It is another limitation of a retrospective study difficult to avoid since patients were follow-up by different nephrologist and some of them decided not to withdraw steroids in younger patients because of the risk of an immunological event and good cardiovascular profile. In older patients, who already had impaired fasting glucose, worse control of blood pressure, dyslipidemia, osteoporosis, etc., the decision of steroids withdrawal was easier to take.
It can be argued that transplants were performed predominantly before 2003 in the group II. We have included all transplant patients who met the rigorous inclusion criteria (especially the maintenance tacrolimus and MMF during the two years of study) of our centre from 1998 to 2011. The protocol steroid withdrawal began in our hospital in 2003, however, it was not applied universally to all patients, for several reasons: acute cellular rejection ≥ IB before 7 months and the presence of several physicians in kidney transplant consultation (refer to the discussion in the previous paragraph). As this group was not broad enough for a non-steroid withdrawal, we extended the study until 1998. During that period, there were patients who had been withdrawn from steroids before 7 months of treatment. This allowed us to have a group of maintenance steroids comparable in time.
Another limitation is the limited sample size, which does not allow reach statistically significant differences. Larger studies with more statistical power are needed to confirm the results.
The inclusion criteria based on the PRA by CDC is another limitation. We detected an increased sensitization level of patients pretransplant by using a more sensitive diagnostic method (Luminex), with a slight tendency towards a higher percentage in group II. We decided not to discard them from the analysis, and to study the effect in these subgroups, although again there were significant differences. When we remove these highly sensitized patients from the study, we observed that the rate of development of HLA-Abs was similar in both groups, making this an important finding from our study.
In conclusion, steroids withdrawal can be carried out safely in terms of HLA-Abs production in those recipients without pretransplant antibodies and under treatment with tacrolimus and MMF. The use of new and more sensitive methods to measure HLA-Abs makes possible to better select patients as candidates for steroids withdrawal.
AuthorsThe authors have participated in the design, conduct or analysis and interpretation of work results. They have reviewed its content and approved the final form of the work.
Conflicts of interestThe authors declare that they have no potential conflicts of interest related to the contents of this article.
FundingThis work was partially supported by grants of Fondo de Investigaciones Sanitarias-ISCIII (PI1100990 and RD16/0009/0027) and Fundación Marqués de Valdecilla-IFIMAV (API 11/24).