Antecedentes: Entre 2004 y 2009, se observó en Argentina un aumento significativo del número de pacientes que iniciaban un tratamiento crónico de hemodiálisis (HD) con una tasa de filtrado glomerular estimada (TFGe) ≥ 10 ml/min/1,73 m2. Métodos: Para su estudio, calculamos las razones de riesgo (RR) de mortalidad en una cohorte de individuos incidentes en HD del Registro Argentino de Diálisis Crónica (2004-2009), que se agrupó, en función de la TFG inicial estimada por CKD-EPI (0-4,9; 5-9,9; 10-14,9; y ≥ 15 ml/min/1,73 m2, siendo 0-4,9 el grupo de referencia), en tres cohortes: «población total», «cohorte sana» (< 65 años sin diabetes ni ningún tipo de comorbilidad) y «cohorte con entrada prevista» (con acceso vascular permanente). Resultados: Tras ajustar los datos de la población (n = 16 931) en función de la edad, el sexo, las enfermedades coexistentes, la albúmina sérica, los ingresos y la existencia de un acceso vascular temporal, se observó una RR de 1,19 (95 % IC: 1,07-1,33) en el grupo con una TFGe ≥ 15 ml/min/1,73 m2. En la cohorte formada por 3897 individuos «sanos», se obtuvieron, tras ajustar las mismas covariables, unas RR de 1,44 (95 % IC: 1,08-1,65) y 1,65 (95 % IC: 1,06-2,55) para los grupos con TFGe iniciales de 10-14,9 y ≥ 15 ml/min/1,73 m2, respectivamente. En los pacientes con «entrada prevista» (n = 6280), tras ajustar los resultados en función de la edad, el sexo, la comorbilidad, el nivel de albúmina sérica y los ingresos, las RR de todos los grupos no difirieron significativamente de las del grupo de control. Conclusiones: Iniciar el tratamiento de HD con una TFGe > 10 ml/min/1,73 m2 no revela ninguna ventaja de supervivencia. La mayor mortalidad del grupo con mayor TFGe que inicia la diálisis es un «artefacto» que está relacionado con una mayor edad, la existencia de más comorbilidades, la hipoalbuminemia y el uso de accesos vasculares temporales.
Background: A significant increase in the number of patients starting chronic hemodialysis (HD) with an estimated glomerular filtration rate (eGFR)≥10mL/min/1.73m2 was observed in Argentina between 2004 and 2009. Methods: In order to study this topic, we calculated the mortality hazard ratios (HR) in a cohort of incident HD individuals from the Argentine Registry of Chronic Dialysis [Registro Argentino de Diálisis Crónica] (2004-2009), grouped according to the initial eGFR (0-4.9, 5-9.9, 10-14.9 and ≥15mL/min/1.73m2 ; reference group 0-4.9) estimated by CKD-EPI; in three cohorts: “total population”, “healthy (<65 years, without diabetes or comorbidities) and “planned entry” (with permanent vascular access). Results: After adjusting the population (n=16,931) for age, gender, coexisting conditions, serum albumin, income, and temporary vascular access a HR of 1.19 (95%CI:1.07-1.33) was observed in the group with eGFR≥15mL/min/1.73m2. In the cohort of 3,897 “healthy” after adjusting for the same co-variates, HRs of 1.44 (95%CI: 1.08-1.65) and 1.65 (95%CI: 1.06-2.55) were obtained for the groups with baseline eGFR values of 10-14.9 and ≥15mL/min/1.73m2, respectively. In “planned entry” patients (n=6,280), after adjusting for age, gender, co-morbidities, serum albumin and income, HRs in all groups were not significantly different as compared to the control group. Conclusions: HD initiation with eGFR>10mL/min/1.73m2 shows no survival advantage. The higher mortality in the group with >eGFR starting dialysis looks like an “artifact” related to higher age, more co-morbidities, low albuminemia and the use of temporary vascular access.
INTRODUCTION
Several nephrology societies advocate the early initiation of dialysis, i.e., when GFR is higher than 10mL/min/1.73m2, to improve the poor outcome of uremic patients on chronic dialysis, despite the lack of supportive evidence. In 1997, the NKF-DOQI suggested to initiate HD with a GFR of 10.0 to 15.0mL/min/1.73m2.1
An updated version of 2006 recommended the initiation with a GFR<15mL/min/1.73m2 in asymptomatic patients and >15mL/min/1.73m2 in symptomatic individuals, considering function levels together with clinical criteria.2 Subsequently, the level for dialysis initiation increased over time in a search for a decrease in mortality.3-8 However, studies from Europe and USA, suggested a lower level of renal function required for the initiation of renal replacement as compared to the one recommended by the guidelines.9-13
Later, other studies from Canada and Australia proposed 12.0 and 10.0mL/min/1.73m2 respectively, with the possibility of defer dialysis in the absence of uremic or malnutrition signs up to a GFR=6ml/min/1.73m2.14-16 Other studies suggested that starting dialysis at higher levels of GFR had no effect on survival17,18 or it could even be counterproductive.19-24
Later, the IDEAL study supported the previous empirical decision to postpone dialysis initiation, as if there were an inverse relation between eGFR and survival.25
Recently, there has been a significant increase in the number of patients initiating dialysis at GFR >10mL/min/1.73m2 in Argentina. It escalated from 29.4% by 2004 to 35% by 2009.26-28
In this project we used a database to retrospectively assess the association between timing of dialysis and survival (in several cohorts) as a primary endpoint, since recent studies in other countries have reported no benefit from early initiation of replacement therapy.
METHODS
This is an observational, retrospective, longitudinal and predictive analysis.
Data source and population
We used data from 32,405 incident patients receiving Chronic HD, gathered from the single forms filed in the Argentine Registry of Chronic Dialysis [Registro Argentino de Diálisis Crónica] from the first day of treatment (this is a mandatory registry). The need for dialysis initiation was always determined by a nephrologist, according to his/her clinical judgment and regardless of the last creatinine value reported to the Registry.
The study included all the patients receiving dialysis for the first time in 462 facilities in the country, from April 1, 2004 to December 31, 2009. These are all of the patients "registered in Argentina" (without statistical bias), approximately 99% of the total number of dialysis patients. Patients younger than 18 years of age (621) and patients who recovered their renal function within the first 30 days of treatment initiation (127) were excluded from the study. In addition, 14,726 patients were also excluded because they were lost to follow-up (3,255) or because they provided incomplete data in the form (11,471) for one of the following variables (clinical, biochemistry and social data): age, gender, diabetes, hypertension, heart failure, arrhythmia, coronary disease, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, neoplasia, body mass index (BMI), hematocrit, albumin, creatinine, HIV, HCV and HB serology, baseline vascular access (native fistulas, arteriovenous prosthesis, permanent and temporary indwelling catheter), financial income, educational level and housing. Before the study, we compared the characteristics of the patients included in the study (16,931) to those excluded due to lost to follow up or incomplete data (14,726), and found that both populations were comparable in terms of age (61.1±15.6 vs 59.6±15.7); diabetes (39.6% vs 38.2%); baseline creatinine value (7.51±4.0 vs 7.77±4.1) and CKD-EPI eGFR (estimated glomerular rate) (8.1±4.6 vs 8.0±4.9); the other available variables were also similar between both populations.
This study includes the 16,931 patients who had completed all variables in the form at the initiation of dialysis. The patients who recovered their renal function after 30 or more days of treatment or who received a transplant were included as censored data. For the analysis, the patients were divided into 3 cohorts: “total population”, “healthy cohort” and “planned entry”.
“Total population”
This population (n=16,931) labeled as “total” was grouped according to the CKD-EPI eGFR (0-4.99; 5-9.99; 10-14.99 and ≥15mL/min/1.73m2 respectively).
“Healthy cohort”
This cohort excluded: patients >65 years old, patients with diabetes, arrhythmia, CHF (chronic heart failure), COPD (chronic obstructive pulmonary disease), peripheral vascular disease, cerebrovascular disorders, cancer, or reactive tests for HIV or HCV. Patients with high blood pressure and a past medical history of ischemic heart disease (unrelated to mortality in this group) were included. Thus, only 3,897 individuals fulfilled this criterion. This cohort was labeled “healthy cohort” and was also grouped according to the initial eGFR obtained using the CKD-EPI equation (4 groups).
“Planned entry” to HD
In an additional exploratory analysis, only data from those starting HD with a permanent vascular access were analyzed (excluding patients starting with a temporary non tunneled catheter). This cohort of 6,280 individuals was labeled “planned entry” and was also grouped according to the initial eGFR obtained from the CKD-EPI equation (4 groups).
Statistical analysis
The Kaplan-Meier product limit estimator and the log-rank test were used to calculate and compare survival among groups. It includes all patients, from the first day of treatment, without any exclusions due to early death.
In order to verify inter-group differences in eGFR among the 3 studied populations, five models of Cox proportional hazard regression with time-dependent covariates to control for potentially confounding factors were implemented. Such approach included a univariate model and 4 multivariate models adjusted for co-variates considered “a priori” to be predictors based on a previous study.29 Finally, the HR from the independent variable (eGFR groups) adjusted for the effect of the remaining independent variables was calculated. The 4 multivariate models are the result of the incorporation of new covariates to the initial model. The eGFR 0-4.99 group was treated as control group in the 3 evaluations. The independent variables closely related among each other (co-linearity) were excluded. Risk proportionality was assessed by log-survival (-log) and the SPSS 15.0 (SPSS Inc. Chicago, IL) statistical package for Windows was used for data analysis.
New age-comorbidity index (NPI): an age-comorbidity score including 19 variables prepared by our group29 similar to Charlson,30 was also applied, showing better predictive results in Argentina.
RESULTS
“Total population”
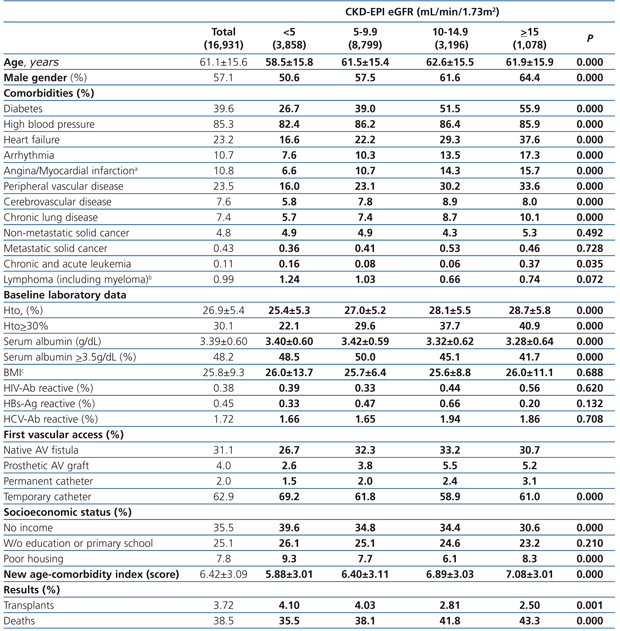
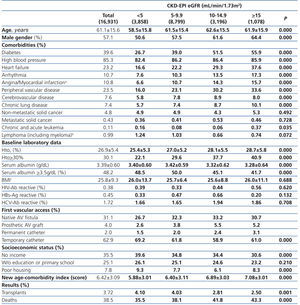
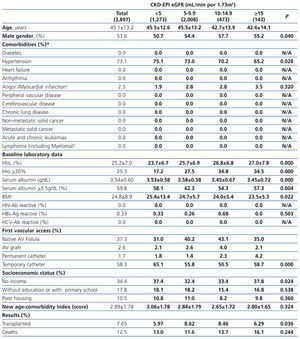
The longest follow-up in this group was of 69.1 months (mean: 20.6±17.9). The baseline characteristics of the study population grouped in 4 different categories based on the eGFR recorded at the time of dialysis initiation are displayed in Table 1. The percentage of individuals with co-morbidities, except for certain conditions such as metastatic and non-metastatic cancer, increases parallel to eGFR at baseline. Hematocrit levels increase with higher eGFRs values, while serum albumin gradually decreases.
The percentage of individuals with a permanent vascular access for HD is significantly higher in the eGFR>5mL/min group and, conversely, the lower the eGFR at the initiation, the higher the percentage of temporary indwelling catheters. The percentage of patients with no income and poor housing is higher with decreasing baseline eGFR levels. Higher baseline eGFR levels are associated with older age, more comorbidities, lower serum albumin and therefore, a higher score in the new age-comorbidity index. In addition, higher eGFRs were accompanied with a higher mortality and lower renal transplant rates.
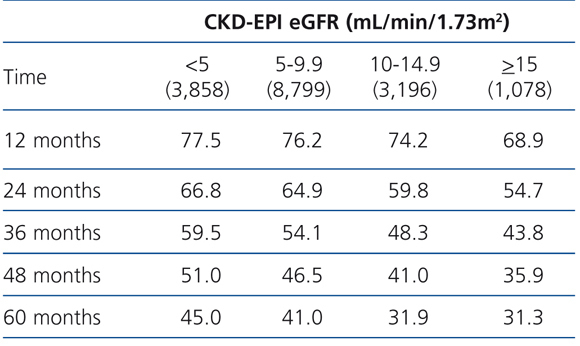
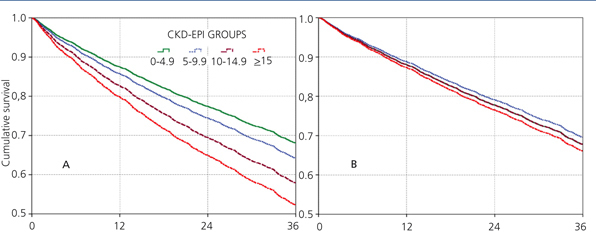
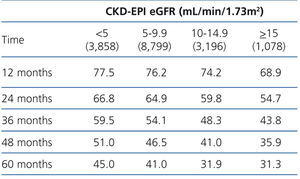
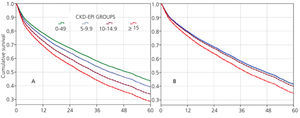
The 60-month unadjusted Kaplan-Meier survival estimate was 45%, 41%, 31.9% and 31.3% for the different eGFR groups (<5mL/min, 5-9.9mL/min, 10-14.9mL/min and ≥15mL/min, respectively) (Table 2: only in “online” edition). The Log-Rank test confirmed a statistically significant difference among them (X2, Chi square test: 83.78 - p=.0001).
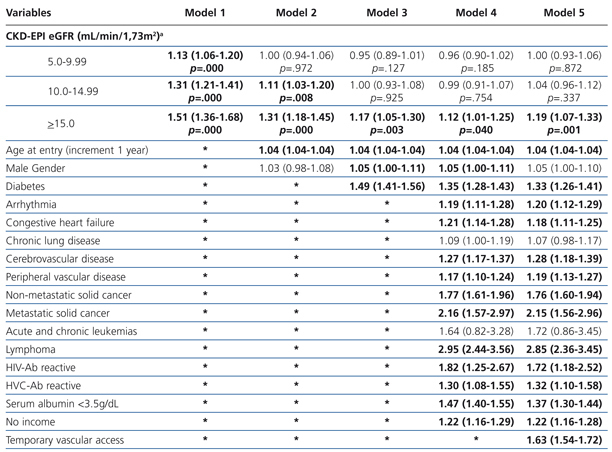
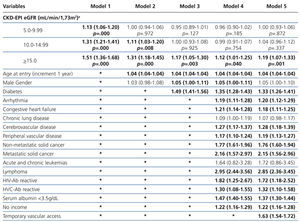
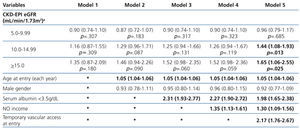
The results from the univariate and multivariate model show (Table 3): as covariates are added to the model, the negative effect on survival (among the higher eGFR groups) gradually decreases, and from model 3, the single group with a significantly elevated HR is that with eGFR≥15mL/min.
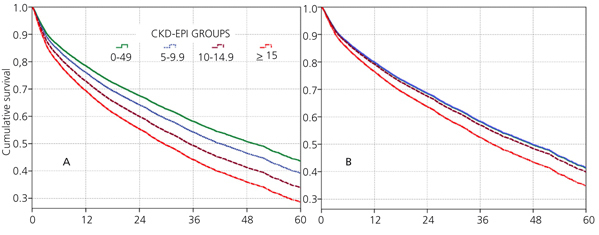
When the covariate “starting HD with a temporary vascular access” is added (model 5), one of the higher HRs is observed in this group (HR: 1.19, 95%CI: 1.07-1.33; p=.001). No differences among the three previous groups could be observed from model 3. The curves for the univariate model as well as for the multivariate model 5 are shown in Figure 1.
“Healthy cohort”
The longest follow-up in this group was 36 months (mean: 21.3±12.9). The baseline characteristics of the cohort of “healthy” individuals recorded at the time of initiating dialysis are displayed in Table 4: only in “online” edition. Except from high blood pressure and a past medical history of ischemic heart disease (unrelated to mortality in this group), these individuals do not suffer from significant comorbidities and no difference is observed in terms of morbidity and transplant rate among the groups.
Similar to what occurs in the “total” population, the percentage of individuals with higher hematocrit levels, lower serum albumin and higher permanent vascular access is higher among those eGFR>5mL/min groups, and, because this special cohort does not suffer from co-morbidities, the NPI score value is very low, with no differences among groups.
The 36-month unadjusted Kaplan-Meier survival estimate was 82.5%, 83.1%, 80.1% and 76.1% for the different eGFR groups (<5mL/min, 5-9.9mL/min, 10-14.9mL/min y ≥15mL/min, respectively – X2 5.92 - p=.116).
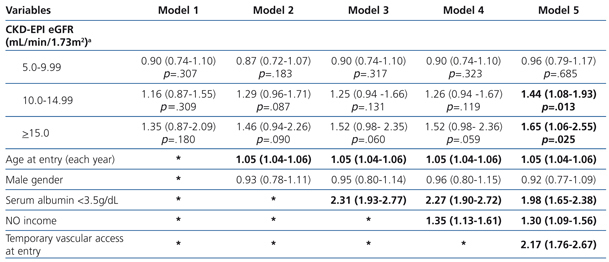
The results from the uni- and multivariate model showed (Table 5: only in “online” edition) that, as covariates were added to the model, no changes in survival among the 4 groups could be observed. Once again, in model 5, in which the covariate “temporary vascular access” was added, the 2 groups with higher eGFRs display significantly higher HRs compared to the reference group (1.44; 95%CI: 1.08-1.93; p=.013 for the 10-14.9mL/min group and 1.65; 95%CI: 1.06-2.55 for the ≥15mL/min group; p=.025).
Cohort with “planned entry”
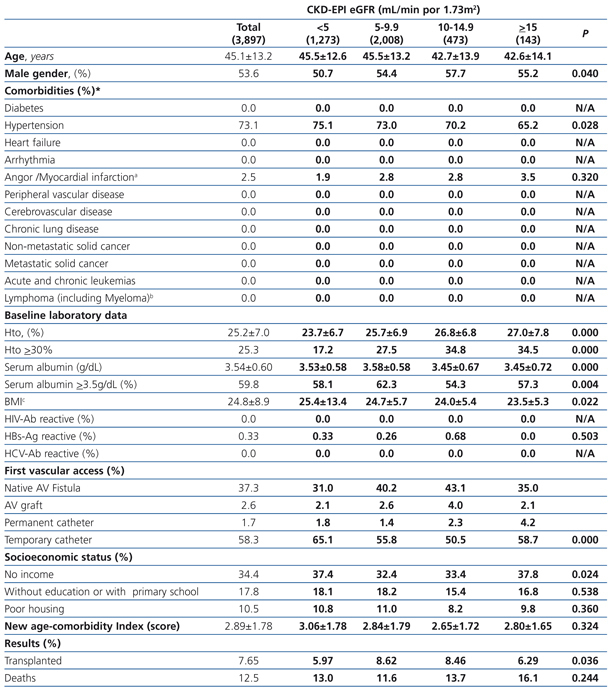
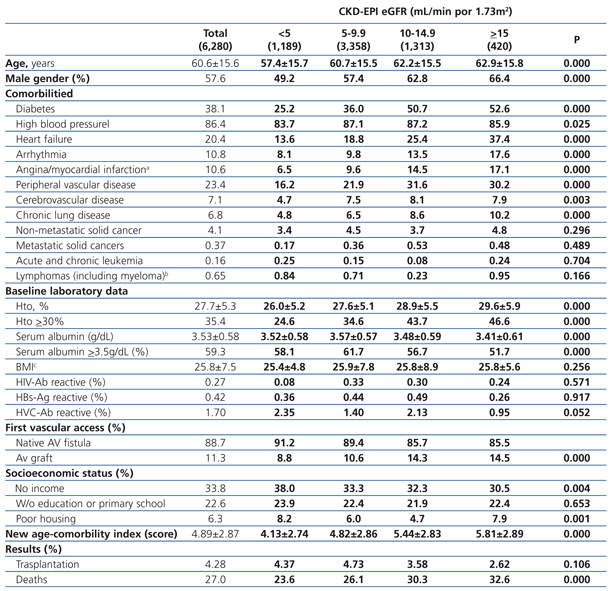
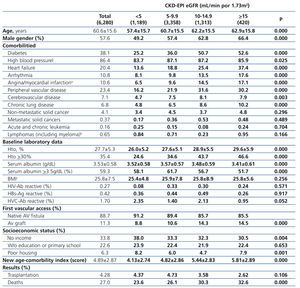
The longest follow-up period in this group was 36 months (mean: 20.7±12.7). The baseline characteristics of the cohort with permanent vascular access recorded at the time of initiating HD are shown in Table 6. Mean age was higher with higher eGFR at the time of initiating HD. The percentage of individuals with co-morbidities (with the exception of those suffering from cancer) increases with higher eGFR values. The hematocrit is also higher in those groups with higher baseline eGFRs, while serum albumin concentration decreases significantly as the eGFR increases. There is a significantly larger proportion of individuals with a native AV fistula in the lower eGFR groups.
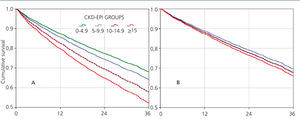
Almost all variables are similar to those observed for the “total population”. The 36-month unadjusted Kaplan-Meier survival estimate was 68.8%, 64.1%, 57.2% and 53.1% for the different eGFR groups (<5mL/min, 5-9.9mL/min, 10-14.9mL/min y ≥15mL/min respectively – X2 38.13 - p=.0001).
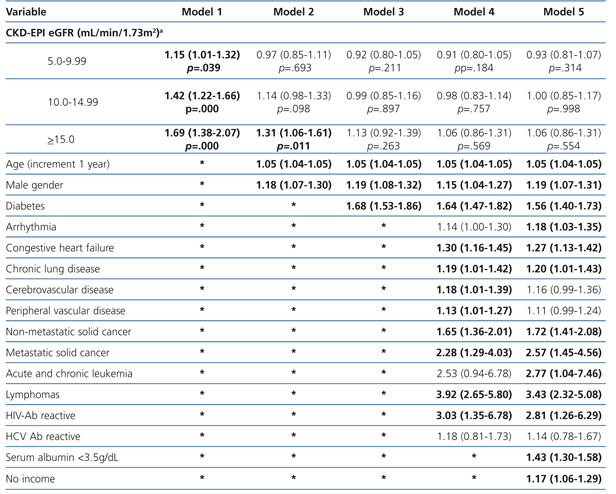
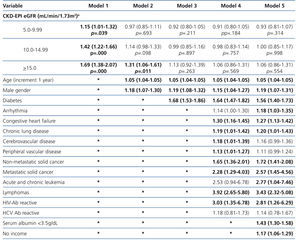
The results from the univariate and multivariate model show (Table 7) that, as compared to the reference cohort, all the groups have a progressively higher HR. As covariates are added to the model, the individual negative effect on survival among groups with higher eGFRs tends to decrease. Adjusting data for age and gender, as occurs in model 2, only the eGFR≥15mL/min group displays a considerable HR value.
No differences among groups compared to the reference cohort are observed in model 3; and no difference in 36-month survival could be detected after adjusting for diabetes, other co-morbidities, serum albumin <3.5g/dL or incomes. In other words, regardless of the eGFR at the time of HD initiation, this will have no influence on 3-year survival provided a permanent vascular access is used. The predictive curves for the unadjusted model 1 as well as for model 5 are shown in Figure 2. In model 5, predictive curves are not significantly different from each other.
DISCUSSION
Traditionally, indicators for dialysis initiation are based on the presence of signs and symptoms of uremia, together with analytical parameters. Guidelines such as those published by the National Kidney Foundation, KDOQI, European Renal Best Practice and Australian and Canadian groups attempt to define and provide a rationale for the answer to questions such as:
When to start dialysis? Numerous observational studies have compared outcomes in patients starting dialysis at various eGFR levels, some with large numbers of patients (>100,000) in registry data from USA, European Registry, French Registry, Canadian Registry and Taiwan Health Insurance.18,31-39
These observational studies show a progressive increase in the mortality rate when dialysis is started with higher eGFR, but they do not constitute conclusive evidence; however, their results should be accepted and accounted for.
There are several possible explanations for the relation between the timing to initiate dialysis and survival:40
a) These studies uses GFR estimates based on the the serum creatinine levels, which present a high level of error when GFR is lower than 30mL/min/1.73m2, also with malnutrition, low muscle mass, hemodilution or frailty, eGFR values appear falsely high. They are “confounding effects” not always corrected for multivariate adjustments. The scarce studies that relate mortality to measured GFR (mGFR) on 24-hour diuresis do not show a positive association between mGFR and mortality (for example: NECOSAD study).11
b) When only the patients who initiated dialysis are considered and excluding those who died before the expected initiation and who were, therefore, not recorded are excluded, a “survivor bias” is created. Only the healthier ones survive long enough to participate in the late initiation. This bias increases in the Taiwan study, that only considered patients who underwent dialysis for more than 90 days. In addition, the combination of the screening bias and the “survival effect”, gives rise to the “immortal time bias”, which improves the survival of the late group as a result of the “survival of the fittest”.
c) Patients with co-morbidity are more likely to be started on dialysis earlier. Multivariate adjustment of this variable decreased, but did not eliminate, the benefit of starting with low eGFR.
d) An additional bias is that related to the management of the initiation time (“lead time bias”), where the measurement does not consider the “extra time” gained delaying the dialysis initiation (artificially improving the early start results).
The Swedish Evans41 study tried to avoid the expectation and survival bias, prospectively enrolling patients when GFR values below 16mL/min/1,73m2: mortality was higher in the early start group.
To eliminate the expectation bias, the Traynor17 study considers survival from the moment the creatinine clearance (CC) estimated by Cockcroft-Gault42 drops below 20mL/min and not from the moment of dialysis initiation. The Traynor study did not show a difference in mortality between the early and late start groups.
The IDEAL study provides evidence that the decision on when to start dialysis is difficult and cannot be guided by any single objective measurement, as 76% of patients assigned to the late group actually started with higher eGRF due to symptoms. The intention to treat with dialysis patients with a CC of 10-14mL/min/1.73m2 (early start) or with a CC of 5-7mL/min/1.73m2 (late start) might have been violated, initiating dialysis in the presence of signs or symptoms, and the final average CC values at baseline were 12 and 9.8mL/min/1.73m2, early and late, respectively. The CC was determined using the Cockcroft and Gault equation corrected by 1.73m2 of BS. A retrospective analysis of the IDEAL study determined eGFR values at early and late start with the 4-variable MDRD equation:43 The levels were 9 and 7.2mL/min, respectively. The randomized, controlled and early planned initiation of dialysis in patients with stage V chronic kidney disease was not associated with an improvement in survival or clinical outcomes.
However, this study, which is considered to be the most relevant trial on the topic of this paper, has certain characteristics that make the comparison with other populations difficult. The great difficulty to separate the groups (early and late initiation), as previously mentioned, is accompanied by the fact that: 56.3% of the patients initiated CAPD (continuous ambulatory peritoneal dialysis) and in most of the records this mode does not exceed the 20%, which adds an additional bias in connection with the election of a therapeutic method. Unlike the IDEAL study, only 4% of the patients entered CAPD first in Argentine (and they were not included in our study). But more importantly, only 6% of the patients in the IDEAL study initiated HD with a temporary catheter as vascular access, which extremely differs from the 45% observed in USA44 and from the 63% corresponding to Argentina. Patients of IDEAL study presented higher Body Mass Index and albuminemia values than our patients or the patients enrolled in USA or Europe.40
Given that urine creatinine and urea measurements from patients included in the Argentine Registry are not available, the GFR was estimated using the CKD-EPI equations45 since MDRD-4 equation employs the age elevated to an exponential expression while this does not happen with the CKD-EPI equation, which makes it possible to obtain linear results regarding age.46 A limitation in our study is that the CKD-EPI equation requires standardized creatinine determination. In Argentina, over 60% of patients receive treatment in health care networks using centralized laboratories with autoanalizer; in any case, calculations made with MDRD-4 do not differ from the ones presented here.
In this study (and in other observational studies), there might be non-measured co-morbidities and other factors affecting survival not included in the analysis. Even after adjusting for comorbidities and other predicting mortality variables, we found in the present study that the patients with eGFR ≥15mL/min have a significantly higher relative risk than that exhibited by those starting dialysis later.
In the multivariate adjustment with several variables that reflect a positive relation with mortality and which are analyzed individually (age, co-morbidities, hypoalbuminemia, poor socio-economic indicators, temporary catheter, etc.), the influence of eGFR on mortality noticeably decreases, but remains significant in the group with eGFR ≥15mL/min/1.73m2.
Retrospective data from the Argentine Registry of Chronic Dialysis from 16,931 patients show that mortality was higher in the group with high baseline clearance, that is to say in early initiation. In the “total population” of this study, an HR of 1.19 (p<.001) is observed in the group with eGFR >15mL/min/1.73m2 at baseline after adjustment for all studied variables.
Then, just like Rosansky et al.,19 we designed a cohort of “healthy” patients to avoid adjustment for predictive co-variates and thus eliminate the “co-morbidities” effect in the final outcome.
We found that the only factor that increases the HR in this group is initiating HD with a temporary indwelling catheter.
Therefore, we decided (via the complete elimination of this factor) to create the “planned entry” cohort, including only the patients who initiate dialysis with a permanent vascular access. Differences are no longer observed and there are no associations between eGFR and survival when patients entering with a permanent vascular access are considered (AV fistula and vascular prosthesis) and adjusted for the variables above.
We then conclude that the relation between eGFR at dialysis initiation and survival is an induced “artifact” since the population entering with a higher estimated filtration is made up of older or sicker patients and since it is associated with an unplanned dialysis initiation through the use of temporary catheters.
Conflict of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Table 1. Baseline characteristics of the total population according to CKD-EPI eGFR at starting HD.
Table 2. KM survival according to CKD-EPI eGFR . &
Table 3. Uni- and multivariate model for Cox proportional hazard in the &
Table 4. Characteristics of the healthy population according to CKD-EPI eGFR at starting HD
Table 5. Uni- and multivariate model for Cox proportional hazard in the healthy population (n=3,897) according to eGFR. Maximum follow-up 36 months
Table 6. Characteristics of the population with planned entry according to CKD-EPI eGFR
Table 7. Uni- and multivariate model for Cox proportional hazard in the population with planned entry (n=6,280) according to eGFR. Maximum follow-up 36 months
Figure 1. Cox proportional hazard regression predictive curves for the &
Figure 2. Cox proportional hazard regression predictive curves for the &