Pulmonary congestion is a strong predictor of mortality and cardiovascular events in chronic kidney disease (CKD); however, the effects of the mild form on functionality have not yet been investigated. The objective of this study was to assess the influence of mild pulmonary congestion on diaphragmatic mobility (DM) and activities of daily living (ADL) in hemodialysis (HD) subjects, as well as compare ADL behavior on dialysis and non-dialysis days. In parallel, experimentally induce CKD in mice and analyze the resulting pulmonary and functional repercussions.
MethodsThirty subjects in HD underwent thoracic and abdominal ultrasonography, anthropometric assessment, lung and kidney function, respiratory muscle strength assessment and symptoms analysis. To measure ADL a triaxial accelerometer was used over seven consecutive days. Twenty male mice were randomized in Control and CKD group. Thoracic ultrasonography, TNF-α analysis in kidney and lung tissue, exploratory behavior and functionality assessments were performed.
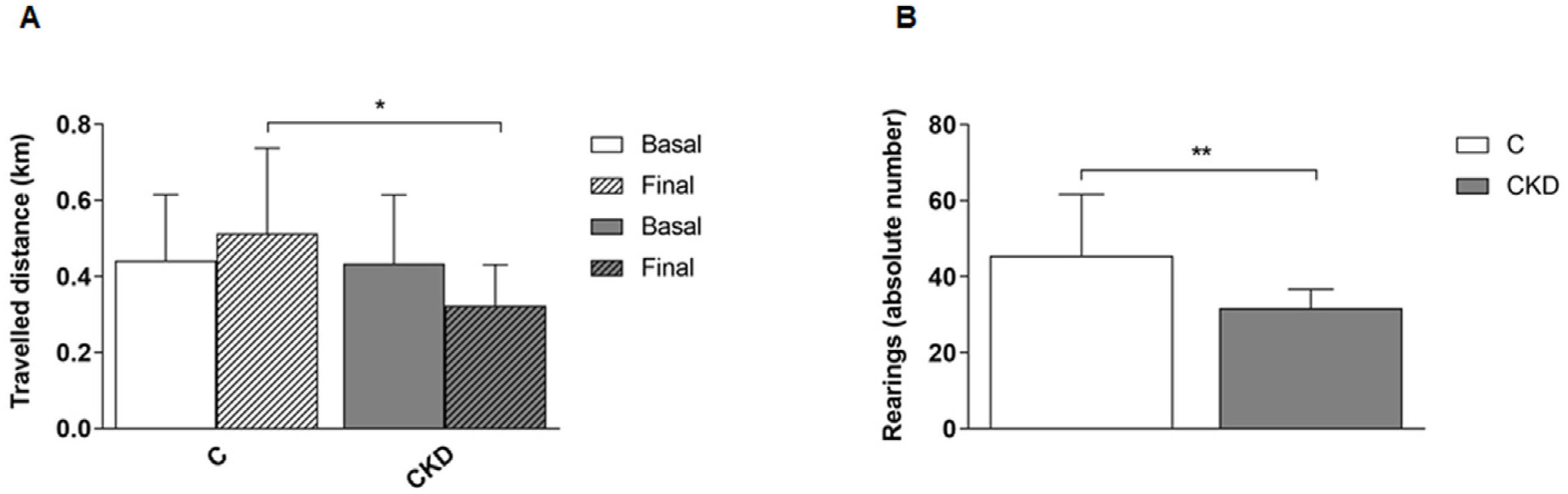
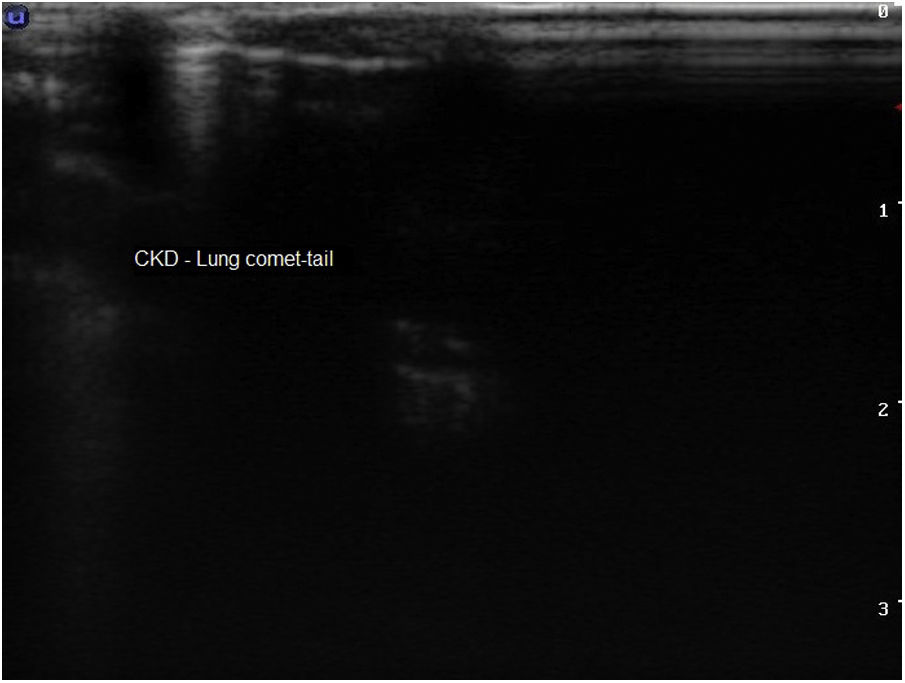
ResultsMild pulmonary congestion caused a 26.1% decline in DM (R2=.261; P=.004) and 20% reduction in walking time (R2=.200; P=.01), indicating decreases of 2.23mm and 1.54min, respectively, for every unit increase in lung comet-tails. Regarding ADL, subjects exhibited statistically significant differences for standing (P=.002), walking (P=.034) and active time (P=.002), and number of steps taken (P=.01) on days with and without HD. In the experimental model, CKD resulted in increased levels of TNF-α on kidneys (P=.037) and lungs (P=.02), attenuation of exploratory behavior (P=.01) and significant decrease in traveled distance (P=.034). Thoracic ultrasonography of CKD mice showed presence of B-lines.
ConclusionThe mild pulmonary congestion reduced DM and walking time in subjects undergoing HD. Individuals were less active on dialysis days. Furthermore, the experimental model implies that the presence of pulmonary congestion and inflammation may play a decisive role in the low physical and exploratory performance of CKD mice.
La congestión pulmonar es un fuerte predictor de mortalidad y eventos cardiovasculares en la enfermedad renal crónica (ERC); sin embargo, aún no se han investigado los efectos de la forma leve sobre la funcionalidad. El objetivo de este estudio fue evaluar la influencia de la congestión pulmonar leve en la movilidad diafragmática (MD) y las actividades de la vida diaria (AVD) en sujetos en hemodiálisis (HD), así como comparar el comportamiento de las AVD en los días de diálisis y no diálisis. Paralelamente, inducir de forma experimental la ERC en ratones y analizar las repercusiones pulmonares y funcionales resultantes.
MétodosTreinta sujetos en HD fueron sometidos a ecografía torácica y abdominal, evaluación antropométrica, función pulmonar y renal, evaluación de la fuerza de los músculos respiratorios y análisis de síntomas. Para medir las AVD se utilizó un acelerómetro triaxial durante 7 días consecutivos. Se aleatorizaron 20 ratones machos en el grupo control y con ERC. Se realizó ecografía torácica, análisis de TNF-α en tejido renal y pulmonar, comportamiento exploratorio y evaluaciones de funcionalidad.
ResultadosLa congestión pulmonar leve provocó una disminución del 26,1% en la MD (R2=,261; P=,004) y una reducción del 20% en el tiempo de caminata (R2=0,200; P=,01), lo que indica disminuciones de 2,23mm y 1,54minutos, respectivamente, por cada unidad de aumento de las colas de cometa pulmonares. En cuanto a las AVD los sujetos mostraron diferencias estadísticamente significativas para estar de pie (P=,002), caminar (P=,034) y tiempo activo (P=,002) y número de pasos dados (P=,01) en los días con y sin HD. En el modelo experimental la ERC resultó en un aumento de los niveles de TNF-α en los riñones (P=,037) y los pulmones (P=,02), la atenuación del comportamiento exploratorio (P=,01) y una disminución significativa en la distancia recorrida (P=,034). La ecografía torácica de ratones con ERC mostró la presencia de líneas B.
ConclusiónLa leve congestión pulmonar redujo la MD y el tiempo de marcha en sujetos sometidos a HD. Los individuos eran menos activos en los días de diálisis. Además, el modelo experimental implica que la presencia de congestión e inflamación pulmonar puede desempeñar un papel decisivo en el bajo rendimiento físico y exploratorio de los ratones con ERC.
The systemic repercussions of the progression of chronic kidney disease (CKD) can compromise the functionality of subjects undergoing hemodialysis (HD), since changes in the respiratory and musculoskeletal systems are common in this population.1–5 Pulmonary congestion is the result of excess fluid, reduced oncotic pressure and greater permeability of the blood-air barrier due to an increase in uremic toxins and inflammatory mediator concentrations, and may lower lung compliance and predispose individuals to restrictive ventilatory defects.3,6 As such, this condition can alter the mechanics of breathing and exacerbate clinical symptoms, compromising the activities of daily living (ADL) of subjects with kidney disease.
Pulmonary congestion is a strong predictor of mortality and cardiovascular events in CKD.6,7 Additionally, previous research has shown that moderate and severe degrees of pulmonary congestion are associated with reduced physical function in subjects undergoing HD and peritoneal dialysis.8,9 However, these studies used self-reporting questionnaires and pedometers, which are known to be inaccurate and limited in terms of assessing ADL. Moreover, the population studied consisted of subjects with moderate and severe degrees of pulmonary congestion, making it difficult to draw conclusions about the functional aspects related to those with milder forms of the condition.
Thus, it is important to investigate the repercussions of mild pulmonary congestion in terms of the respiratory mechanics and functionality of these individuals. The aim of this study was to assess the influence of mild pulmonary congestion on diaphragmatic mobility (DM) and ADL in HD subjects, as well as compare ADL behavior on days with and without HD. Besides that, to provide a better understanding of the possible mechanisms involved in this process by performing an experimental research with adenine-induced CKD animal model.
MethodsClinical studyThe clinical research was approved by the Research Ethics Committee of the Universidade do Estado de Santa Catarina (CAAE: 34247814.9.0000.0118) and used a descriptive, observational cross-sectional design. All the participants gave written informed consent. Inclusion criteria were (1) clinical diagnosis of CKD and undergoing HD for at least six months; (2) under medical supervision and not suffering from any other acute disease; (3) no recent diagnosis (less than three months) of ischemic heart disease, unstable angina, severe heart arrhythmia, respiratory, orthopedic and neurological diseases; and (4) not participating in physical training programs within the last 6 months. Individuals were excluded from the study when they (1) exhibited pulmonary congestion classified as moderate or severe (>14 lung comet-tail); (2) were unable to perform any of the study assessments (lack of understanding or cooperation); or (3) displayed decompensation of the clinical picture during assessment.
A convenience sample consisted of 30 subjects with CKD, who performed HD three times a week. The data was collected at Associação Renal Vida in Blumenau, Santa Catarina, Brazil. Assessments were performed on two different days, before the HD sessions. On the first day, individuals underwent thoracic and abdominal ultrasonography (USG) to assess pulmonary congestion and DM, respectively. On day two, they were evaluated in terms of anthropometric data, lung and kidney function, and respiratory muscle strength, and their symptoms analyzed based on the New York Heart Association Functional Classification (NYHA scale). Next, they were instructed to use a triaxial accelerometer over seven consecutive days for 12h a day, to measure ADL-related outcomes.
Pulmonary congestion assessmentPulmonary congestion was assessed by a radiologist using ultrasound (HDI-5000®; Philips, Eindhoven, Netherlands) with a 5MHz convex transducer. The assessment was performed with the individual in the supine position. The anterior and lateral regions of the chest wall were evaluated bilaterally, from the second to fourth intercostal space in the left hemithorax, and from the second to fifth intercostal space in the right hemithorax. The presence of lung comet-tails was observed in each intercostal space, considering four zones (parasternal, midclavear, anterior axillary and midaxillary), resulting in the observation of 28 lung zones. These lung comet-tails, also known as B-lines, are ultrasound artifacts caused by thickened subpleural septa due to the presence of extravascular lung water. Based on the total number of lung comet-tails, pulmonary congestion was classified as mild (up to 14), moderate (15 to 30) and severe (over 30).2,9,10
DM assessmentDM was evaluated by a radiologist using B-mode ultrasound (HDI-5000®; Philips, Eindhoven, Netherlands) with a 5MHz convex transducer. DM was measured indirectly, with subjects in the supine position and their arms at their sides, by craniocaudal displacement of the left branch of the portal vein, considering the distance between points during maximal inspiration and expiration.11,12 Three measurements were taken and the largest was used for analysis.
ADL assessmentADL were assessed using a triaxial accelerometer (DynaPort MiniMod®; McRoberts BV, The Hague, Netherlands). Individuals were instructed to begin using the device immediately after waking up, for 12h a day over seven consecutive days.13 They were also advised not to change their habitual daily activities while using the device. The variables used for analysis were time spent lying down, sitting and standing, sedentary time (time spent sitting and lying down), walking time, active time (walking and standing) and number of steps taken.
Anthropometric assessment and kidney function indicatorsWeight and height were measured on a calibrated scale and stadiometer, respectively. Next, subjects were classified based on their body mass index (BMI) as underweight (<18.5kg/m2), eutrophic (18.5–24.9kg/m2), overweight (25–29.9kg/m2) and obese (≥30kg/m2).14 The glomerular filtration rate (GFR), Kt/V ratio, calcium, phosphorus, serum iron, urea, creatinine, and albumin values were taken from individuals’ most recent monthly medical examination.
Lung function assessmentLung function was evaluated with a previously calibrated portable digital spirometer (EasyOne®; NDD, Medical Technologies, Andover, MA, USA), in accordance with the recommendations of the American Thoracic Society and European Respiratory Society.15 The variables analyzed were forced vital capacity (FVC), forced expiratory volume in one second (FEV1) and the FEV1/FVC ratio, all expressed as absolute values and percentages of predicted normal values.16 Maximal voluntary ventilation (MVV) was also expressed in absolute values and percentages of predicted normal values.17 Normal lung function was classified as FVC and FEV1≥80% of the predicted value and FEV1/FVC≥0.7. Subjects who exhibited values below normal underwent spirometry again after inhaling a bronchodilator.
Respiratory muscle strength assessmentRespiratory muscle strength was measured using a digital manometer (MVD300®; Globalmed, Porto Alegre, Brazil), according to the guidelines of the Brazilian Pulmonology and Thoracic Society.18 The largest value obtained during maximal inspiratory (MIP) and expiratory pressure (MEP) maneuvers was considered for analysis, expressed as an absolute value and percentage of the predicted normal value.17
Functional classification of symptomsSymptoms were functionally classified (NYHA scale) in order to place subjects in one of four categories based on how much they were limited during physical activity: I – no symptoms during ordinary physical activity, with limitations similar to those expected in healthy individuals; II – mild symptoms during ordinary activity; III – marked symptoms even during less-than-ordinary activity; IV – symptoms present at rest.19
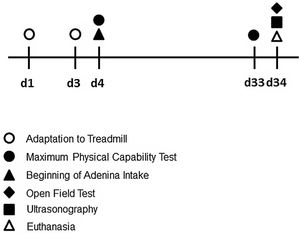
Experimental studyThe experimental research was submitted to the Ethics Committee on Animal Use (CEUA) of the Universidade Federal de Santa Catarina (no. 9256210519) and to the Universidade do Estado de Santa Catarina (no. 2306201018). All the animals received humane care in compliance with EU Directive 2010/63/EU and Animal Research: Reporting In Vivo Experiments (ARRIVE), which provides guidelines for animal experiments. Twenty male Swiss mice (30–40g), young adults, aged 8–10 weeks, were maintained in controlled conditions for temperature (22±2°C), humidity (70–75%) and dark/light cycle (12h; lights on at 07:00 am). The diet consisted of water and ration ad libitum. The animals were randomized in 2 groups: Control (C, n=10) and Chronic kidney disease (CKD, n=10). The sequence of CKD induction, testing period and euthanasia are illustrated in Fig. 1.
Experimental model of adenine-induced CKDCKD was induced by adding 0.2% adenine (SIGMA®) (i.e. 0.2g adenine/100g feed) to the mice diet for four weeks, following the model established by Ali et al. (2013).20 The Control group animals, in turn, received the conventional ration (BioBase®, BioTec line, Chapecó, Santa Catarina, Brazil).
Maximum physical capability test (MPCT)All the animals were adapted to the treadmill (Advanced 2, Athletic®, Santa Catarina, Brazil) for three days, performing 10min of activity at a speed of 0.2km/h. Twenty-four hours after adaptation, all the mice were submitted to the MPCT. The MPCT, adapted from Vieira et al. (2007)21 was performed with five minutes of heating at 0.2km/h, followed by an increase in velocity of 0.1km/h each 2.5min, until animal exhaustion, observed when mice could no longer run, even after 10 small mechanical stimuli. The travelled distance (km) for each animal was recorded.
Exploratory and locomotor activityOpen Field Test (OFT) was performed to evaluate the exploratory locomotor activity of mice. Each mouse was placed alone in an open field arena (40×340m) and animal behavior was recorded during five minutes by a digital camera. The images were analyzed by Any-maze software in a blinded fashion. We evaluated the number of rearings (exploratory movement in which the animal stands).22
Thoracic USGAnimals were put under anesthesia with isoflurane 1–2%, through a face mask and positioned in supine. A triplex scanning ultrasound device (Logiq 3, General Electric Company, Milwaukee, Wisconsin, 53201, United States of America), with a 6–10MHz linear array probe, was used to assess for the presence of pulmonary congestion.23
Blood collection and analysisAfter performing the thoracic USG, the animals were anesthetized with xylazine and ketamine. A surgical incision was made in the midline of the animals’ abdomen, and a blood sample from the abdominal aorta artery was collected in heparinized syringes with approximately 1mL. Samples were centrifuged (4000rpm, at 4°C, for 15min) and the resulting serum aliquoted and stored at −80°C for further analysis of plasma creatinine and urea concentrations by spectrophotometry using commercial kits. After blood collection, animals were euthanized by arterial section.
Analysis of TNF-α in kidney and lung tissuesKidney tissue samples were homogenized and the supernatant stored at −80°C. Total protein was measured in the supernatant using the Bradford method, and levels of TNF-α (R&D Systems – Minneapolis, MN, United States of America) were analyzed, according to manufacturer's instructions. Tissue cytokine levels were estimated by interpolation from a standard curve using colorimetric measurements at 450nm (540nm wavelength correction) on an ELISA plate reader (Berthold Technologies – Apollo 8 – LB 912, KG, Germany). The results of the tissue samples were expressed as picogram per milligram (pg/mg).
Lung histologyThe left lung of each animal was separated in order to prepare slides stained with Picro-Sirius Red, for qualitative assessment of collagen fibers content.
Statistical analysisNormal distribution was determined using the Shapiro-Wilk test. Anthropometric data, kidney and lung function, respiratory muscle strength, DM, pulmonary congestion, ADL and symptom classification were presented as absolute frequencies and measures of central tendency and dispersion. Spearman's correlation coefficient was used to assess the relationship between pulmonary congestion and the other study variables. The correlation coefficient was classified, such as: low (.10–.39), moderate (.40–.69) and high (.70–1.00).24 Simple linear regression was performed to determine the influence of mild pulmonary congestion on DM and ADL outcomes. Finally, a paired t-test and the Wilcoxon test were applied to compare the ADL outcomes of subjects on days with and without HD, also for comparisons between the experimental groups. Significance was set at 5%. The statistical program GraphPad Prism 8 was used for all the analyses.
ResultsOf the 30 subjects with CKD studied, 56.6% were women and approximately 50% were classified as eutrophic based on their BMI (Table 1). All the participants were under conventional HD.
Anthropometric data and kidney function indicators of the subjects assessed.
| Parameter | n=30 |
|---|---|
| Anthropometric data | |
| Gender (M/F) | 13/17 |
| Age (years) | 49.47±15.48 |
| Weight (kg) | 70.07±22.76 |
| Height (m) | 1.62±0.09 |
| BMI (kg/m2) | 26.64±7.37 |
| Kidney function indicators | |
| GFR (mL/min/1.73m2) | 5.98±4.67 |
| Kt/V | 1.51±0.40 |
| Urea (mg/dL) | 148.50±40.80 |
| Creatinine (mg/dL) | 9.90±3.48 |
| Albumin (g/dL) | 3.78±0.68 |
| Iron (μg/dL) | 94.10±40.57 |
| Calcium (mg/dL) | 8.72±0.50 |
| Phosphorus (mg/dL) | 94.10±41.27 |
M: male; F: female; BMI: body mass index; GFR: glomerular filtration rate; Kt/V: Kt/V ratio. Data expressed as mean±standard deviation.
Participants exhibited a decline in percentages of predicted values for the spirometric variables (FVC, FEV1 and MVV), and reduced inspiratory muscle strength when compared to predicted values. No subjects displayed obstructive disorders in the lung function assessment. The mean of DM was 59.76mm and a mean of 7.13 lung comet-tails were observed. Half of the participants were classified as category I on the NYHA scale, demonstrating that their symptoms did not limit their daily activities. However, the other 50% of the sample reported limiting symptoms even when presenting less severe pulmonary congestion (Table 2).
Data on lung function, respiratory muscle strength, functional classification of symptoms, pulmonary congestion, DM and ADL for the subjects assessed.
| Parameter | n=30 |
|---|---|
| Lung function | |
| FEV1/FVC | 0.81±0.02 |
| FVC (L) | 2.55±0.99 |
| FVC (%) | 71.07±18.07 |
| FEV1 (L) | 2.07±0.82 |
| FEV1 (%) | 67.53±21.00 |
| MVV (L) | 71.94±29.01 |
| MVV (%) | 53.20±14.54 |
| Respiratory muscle strength | |
| MIP (cmH2O) | 74.10±32.75 |
| MIP (% Predicted) | 60.20±27.73 |
| MEP (cmH2O) | 82.77±29.30 |
| MEP (% Predicted) | 82.12±27.38 |
| Functional classification of symptoms | |
| NYHA (I/II/III/IV) | 15/9/3/3 |
| Pulmonary congestion | |
| Lung comet-tails (no.) | 7.13±3.73 |
| Diaphragmatic mobility | |
| DM (mm) | 59.76±1.63 |
| ADL (average over 7 days) | |
| Time spent sitting (min) | 314.53±85.34 |
| Time spent lying down (min) | 287.32±98.31 |
| Sedentary time (min) | 601.85±35.62 |
| Standing time (min) | 86.70±29.82 |
| Walking time (min) | 34.61±12.83 |
| Active time (min) | 121.32±35.21 |
| Steps taken (no.) | 3710.93±1373.62 |
FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; MVV: maximal voluntary ventilation; MIP: maximal inspiratory pressure; MEP: maximal expiratory pressure; DM: diaphragmatic mobility; NYHA: New York Heart Association; ADL: activities of daily living. Data expressed as mean±standard deviation.
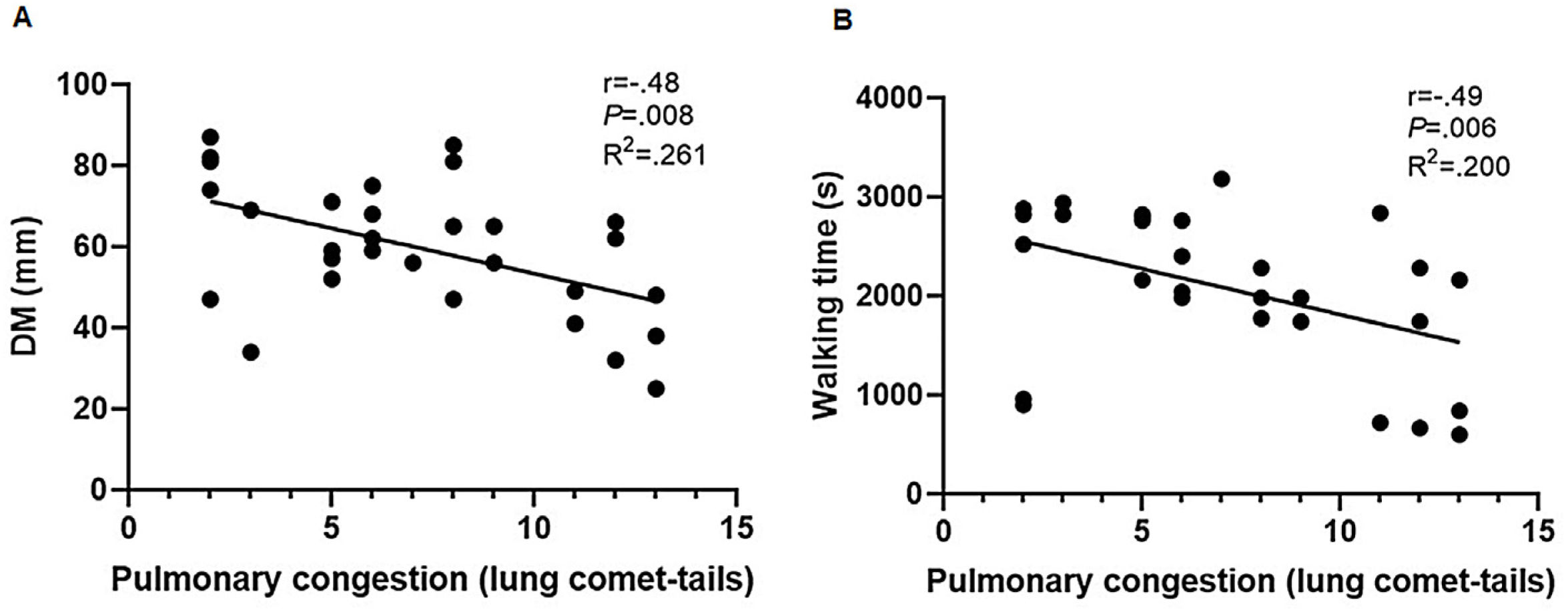
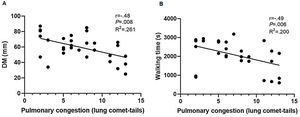
There was a moderate correlation between pulmonary congestion and DM (r=−.48; P=.008). Simple linear regression revealed that mild pulmonary congestion reduced DM by 26.1% (R2=.261; P=.004), indicating a 2.23mm decrease in DM for every unit increase in lung comet-tails (Fig. 2A). A moderate correlation was also observed for pulmonary congestion and walking time (r=−.49; P=.006). Simple linear regression revealed that mild pulmonary congestion decreased walking time by 20% in HD subjects (R2=.200; P=.01), indicating a 1.54-min decline for every unit increase in lung comet-tails (Fig. 2B).
Regarding the ADL behavior, no statistical difference was observed between the variables time spent sitting (328.18±128.85min vs. 322.98±111.42min; P=.86), time spent lying down (295.3±142.52min vs. 274.15±130.6min; P=.44) and sedentary time (623.49±46.8min vs. 597.13±76.38min; P=.10) on days with and without HD, respectively. The statistically significant differences were observed only for the variables standing time, walking time, active time and number of steps taken (Table 3).
Comparison of ADL behavior on days with and without HD.
| Parameter | With HD | Without HD | P |
|---|---|---|---|
| Standing time (min) | 75.51±37.45 | 109.76±51.23 | .002* |
| Walking time (min) | 39.5±21.93 | 51.68±27.53 | .034* |
| Active time (min) | 115.01±53.54 | 161.45±65.25 | .002* |
| Steps taken (no.) | 3286.8±1798.94 | 4422.86±2202.53 | .01* |
Data expressed as mean±standard deviation.
Parallel to these findings, the experimental study also showed corresponding results, since there was a significative impairment of creatinine clearance in CKD group, as described in Table 4.
Clearance of creatinine in experimental groups.
| Parameter | Control group (n=6) | CKD group (n=7) |
|---|---|---|
| Urinary flow (mL/min) | 0.020±0.001 | 0.030±0.002* |
| UCr (mg/dL) | 49.50±21.06 | 13.00±5.44* |
| SCr (mg/dL) | 0.39±0.11 | 0.67±0.19* |
| CrCl (mL/min) | 3.76±0.57 | 0.70±0.14* |
CKD: chronic kidney disease; UCr: urinary creatinine; SCr: serum creatinine; CrCl: creatinine clearance. Data expressed as mean±standard deviation.
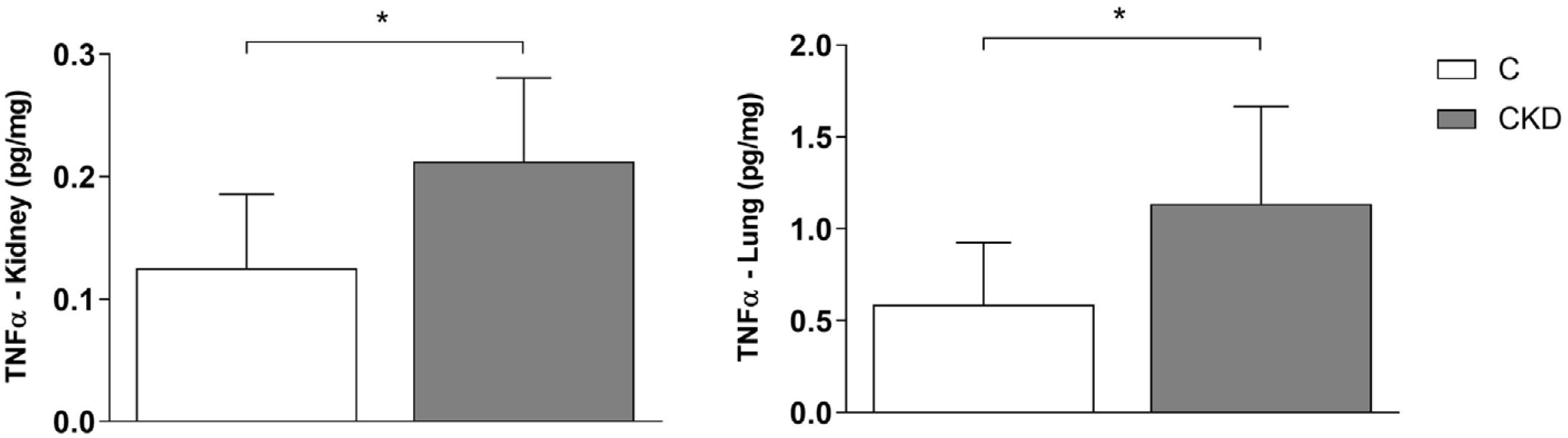
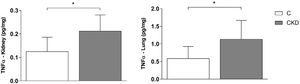
The CKD group also showed a significant increase of TNF-α in both kidney (P=.037) and lung (P=.02) tissues, compared to the Control group (Fig. 3).
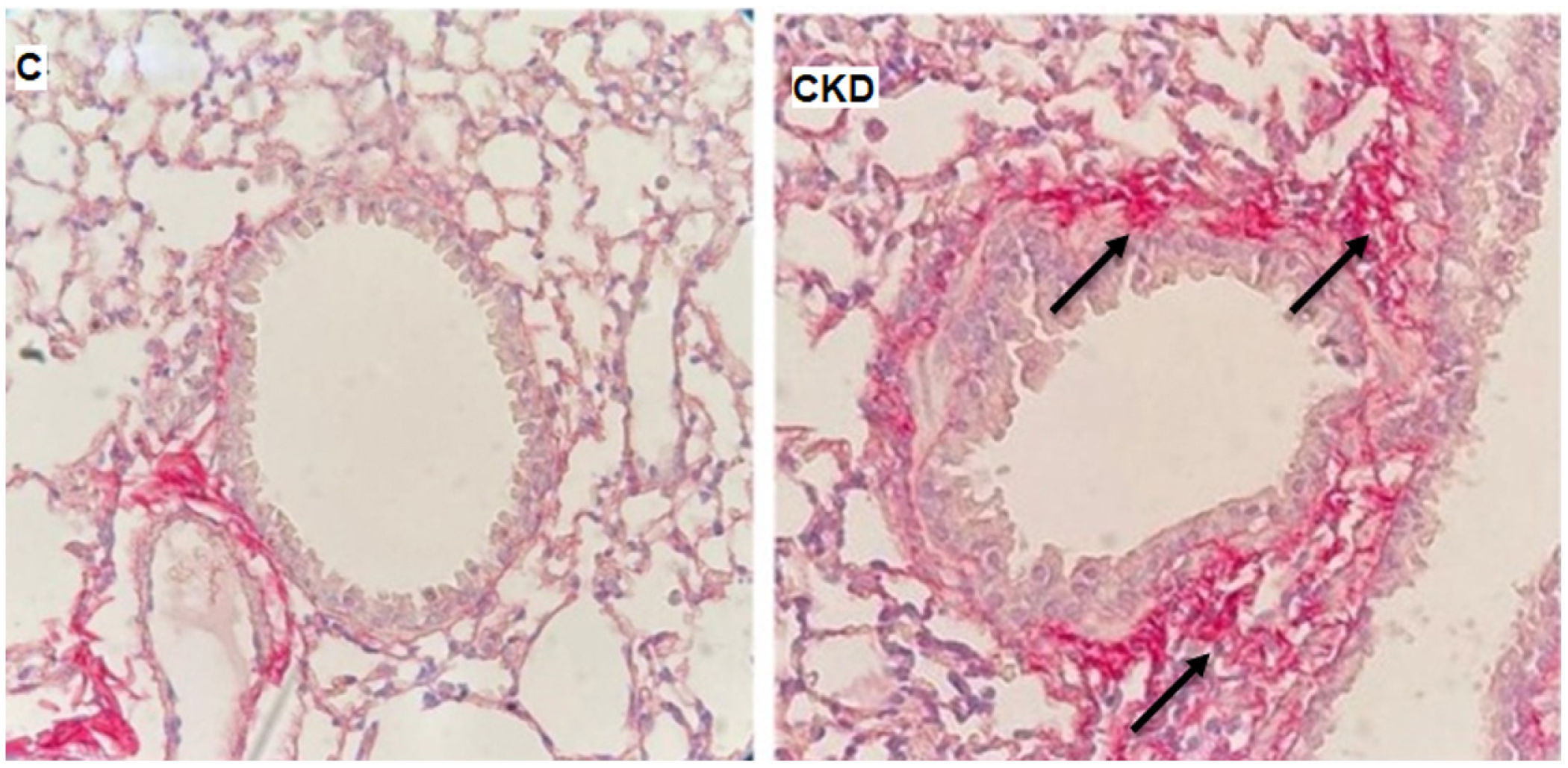
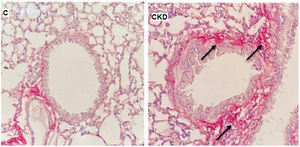
In order to ratify the lung impairment, we also evaluated the histological pattern of adenine-induced CKD mice, and our findings on a qualitative increase in collagen content in lungs of CKD group, as shown in Fig. 4.
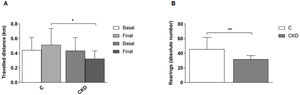
The physical capacity of the animals was assessed by the maximum travelled distance (km) during the MPCT. After adenine induced CKD, the animals in the CKD group showed a significant decrease in the travelled distance (P=.034) when compared to the Control group (Fig. 5A). The exploratory and locomotor activity were evaluated by the OFT, the absolute number of rearings over five minutes was recorded as an indirect measure of functionality. This assessment is related to the animal's ability to support its own weight on its legs (Fig. 5B). After adenine intake, the CKD group had a statistically significant reduction in the number of rearings when compared to the Control group (P=.01).
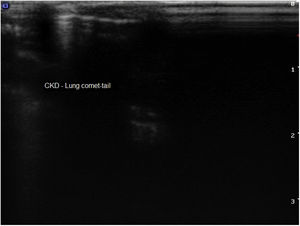
Finally, thoracic USG was able to identify the presence of B-lines in the lungs of CKD mice, a strong indication of CKD-associated pulmonary congestion (Fig. 6).
DiscussionThe main finding of our study was that even low levels of fluid buildup in the lungs can compromise DM and ADL performance in HD individuals. In the experimental context, although it is not possible to evaluate the grade of pulmonary congestion through USG in small animals, it was possible to identify the presence of B-lines, and based on this it may be suggested that its presence may have played a decisive role on poor physical performance and exploratory behavior of adenine-induced CKD mice.
In our sample, 26% of DM was influenced for the mild pulmonary congestion. Although there was no apparent change in DM in the subjects studied here, there are no reference values in the literature for this outcome, making it difficult to confirm. However, studies that assessed DM in healthy individuals during deep breathing via USG found average values close to 60mm,25,26 similar to the 59.76mm recorded here. Although diaphragm dysfunction was not present, mild pulmonary congestion was capable of compromising diaphragm movement, likely due to the negative effects of fluid buildup, such as a decline in lung compliance, volumes and capacities caused by restrictive ventilatory defects.3,6 Thus, because the participants exhibited only mild pulmonary congestion, we hypothesize that more severe forms of the condition may exert an even greater influence due to increased lung restriction. Applying clinical and physical therapy techniques to reduce excess fluid accumulation would therefore be a relevant strategy in this population. Since our study is the first to investigate the effects of pulmonary congestion on DM, further research is needed to better understand changes in the respiratory mechanics in CKD.
Our results suggest that ADL may be compromised in CKD subjects with mild pulmonary congestion, because walking time was influenced in 20% by fluid buildup in the lungs. In parallel, MPCT was performed by mice in this study, as a measure of functionality, and the animals presented a significant reduction in the maximum travelled distance. Another measure that can be associated with ADL in humans is the number of rearings performed by the animals at OFT, which also showed a significant reduction. Similar results were reported by Enia et al. (2013)8 in individuals with moderate and severe pulmonary congestion, whereby an increase of 10 lung comet-tails raised the probability of worse physical performance by 23%. In light of these findings, our results reinforce the fact that even mild pulmonary congestion can compromise the functionality of HD subjects. As such, introducing interventions aimed at minimizing the effects of pulmonary congestion would allow for greater improvement in functional capacity in these individuals, even for milder forms of the condition.
The ADL behavior of these subjects on dialysis and non-dialysis days was also compared. A statistical difference was observed for the variables standing, walking and active time, and number of steps taken, whereby individuals exhibited an increase in active behavior on non-dialysis days. Several authors have used questionnaires and equipment to estimate energy expenditure or number of steps taken per day and observed sedentary behavior and inactivity in this population when compared to healthy individuals.27–32 Additionally, our results corroborate those reported by Gomes et al (2015),33 who found that subjects took fewer steps, walked less, and spent less time standing and more lying down on non-dialysis days, largely due to the period of inactivity during dialysis. There are reports in the literature that these subjects are 24% less active on dialysis days, with the treatment itself responsible for this decine.27 These findings indicate that dialysis subjects tend to be sedentary, particularly on treatment days, making it important to provide physical training programs during HD in order to improve their activity levels.
With respect to pulmonary function, the results obtained corroborate those reported in the literature.1,34,35 Approximately 67% of participants (n=20) displayed some form of restrictive ventilatory defect in the pulmonary function test, demonstrating that CKD subjects tend to experience limited ventilation due to excess fluid accumulation and respiratory muscle dysfunction.1,36 Moreover, our sample also exhibited a decline in FEV1 percentage, but no obstructive lung disorders. Previous studies have suggested that reduced FEV1 in these individuals is the result of fluid overload near the small airways, hampering breathing.34,37
In regard to respiratory muscle strength, mean MIP was below predicted normal values. Participants also showed a 50% decline in predicted normal MVV values, a spirometric variable related to muscle endurance. Thus, our individuals exhibited a reduction in both inspiratory muscle strength and respiratory muscle endurance. Previous research reports that decreased muscle strength in CKD is the result of factors such as systemic inflammation, metabolic acidosis, hormonal changes and nutrient deficiencies, which occur as the disease progresses and culminate in reduced muscle mass, strength and endurance, condition known as sarcopenia.5,36 The experimental data supports this information, since levels of TNF-α were elevated both in kidney and lung tissues, reflecting this state of chronic inflammation and probably influencing on the reduction in respiratory muscle strength and endurance. Previous studies with the elderly and subjects with chronic obstructive pulmonary disease have found a reduction on diaphragmatic thickness and MIP when these conditions were associated with sarcopenia.38,39 Based on this, sarcopenia in CKD, in addition to affecting the peripheral musculature, could compromise the respiratory musculature, contributing to a reduction in lung capacity and the emergence of dyspnea in this population.1,3
The symptoms of half of the participants studied here were categorized as class I on the NYHA scale and 20% fell under classes III and IV. This suggests that a small portion of individuals with mild pulmonary congestion may already experience significant symptoms that limit their daily activities. Salerno, Parraga and Mcintyre (2017)3 reported that dyspnea may occur in CKD subjects due to the emergence of pulmonary congestion, which reduces lung compliance, restricts lung parenchyma and hampers gas exchange across the blood-air barrier. This, combined with impaired respiratory muscles, can further exacerbate the disease.40
Our study was innovative in that it investigates the influence of pulmonary congestion on functional outcomes and respiratory mechanics in a group of HD individuals typically excluded from research for exhibiting a less severe form of the condition. The intention of running an experimental set in parallel was to provide background and some extra information to justify the findings and make them more robust. A limitation in the translation of data was not being able to ensure the mild level of pulmonary congestion in the animals, only to prove the presence of it. The animals were not at the end-stage of renal disease, so they did not need HD, and we were not able to perform pulmonary mechanics measurements, like DM. Regarding clinical data, residual diuresis and interdialytic weight gain are variables that can influence the function of the cardiac muscle and the pulmonary fluid build-up in our population. Thus, not measuring these variables can be considered a limitation in our study. The lack of knowledge about the prevalence of sarcopenia can be considered as another limitation, making it difficult to understand the negative effects of this dysfunction on the respiratory muscles of CKD subjects. Another limitation of the study is that we did not use fluoroscopy, which is capable of evaluating DM in real time. However, it is important to underscore that indirect DM measurement by craniocaudal displacement of the left branch of the portal vein has proved to be a practical, valid and reproducible technique.11,12 Failure to measure excursion of the left hemidiaphragm is another limitation, but a previous study indicated no statistical differences between right and left DM measurements,41 making it easier to measure this outcome in clinical practice.
In spite of these limitations, the results of the present study demonstrate the importance of assessing and monitoring subjects in HD even when they exhibit only mild pulmonary congestion. Although the pathophysiology of CKD differs from that of chronic lung and heart diseases, its progression has similar systemic repercussions that can compromise functional capacity and increase sedentary behavior and cardiovascular-related mortality in this population. In this sense and as previous studies have reported better outcomes in CKD individuals undergoing aerobic exercise, peripheral and respiratory muscle training,42,43 structured rehabilitation programs need to be encouraged and implemented during HD.
ConclusionThe present findings allow us to infer that mild pulmonary congestion reduced DM and walking time in subjects undergoing HD. The ADL behavior differed in this population on days with and without HD, with individuals being less active on dialysis days. Furthermore, the experimental model implies that the presence of pulmonary congestion and inflammation may play a decisive role in the low physical and exploratory performance of CKD mice.
FundingThe study was funded by the Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) (no. 2017TR645).
Conflicts of interestThe authors have disclosed no conflicts of interest.