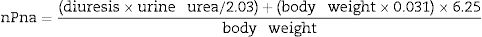A controlled protein intake has shown beneficial effects to preserve renal function and nutritional status in chronic kidney disease (CKD) patients. This study aimed to analyze usual dietary protein intake and its potential contribution to body composition in CKD patients in stages 3–5.
MethodCross-sectional study in 134 CKD patients in stages 3–5 (mean e-GFR: 19.4±8.7ml/min/1.73m2; males 68.7% and primary CKD etiology was diabetes mellitus, 35.8%). Demographic, clinical and nutritional parameters were evaluated. Normalized protein nitrogen appearance (nPNA), was used as a surrogate marker of dietary protein intake. The sample was classified into three nPNA groups (Gn): G1: <0.8g/kg/day; G2: 0.8–1g/kg/day and, G3: ≥1g/kg/day. Assessment of nutritional status using the malnutrition-inflammation score (MIS), anthropometric measures and laboratory parameters. Analysis of body composition and hydration status by bioelectrical impedance analysis (BIVA-101-RJL system). Statistical analysis by SPSS v.20.
ResultsOverall mean nPNA values were 0.91±0.23g of protein/kg BW/day and only 32.1% had a dietary protein intake <0.8g of protein/kg BW/day. Most of the CKD patients (65.5%) were in stages 4 or 5. Prevalence of protein–energy–wasting (PEW) syndrome measured by MIS was 15%. By analyzing differences between nPNA groups, body weight (BW), BMI and triceps-skinfold (TSF) thickness were significantly higher in the group with nPNA ≥1g/kg BW/day (G3), whereas a significant inverse relationship was found with the percentages of body cell mass (BCM%), fat-free mass (FFM%), muscle mass (MM%) and phase angle (PA) in the group with the lowest nPNA (G1). Analysis of gender among subjects showed significant differences with BW, FFM%, TSF and mid-arm muscle circumference (MAMC%). Linear regression analysis showed that resistance, BCM%, MM%, and serum albumin were significant predictors of nPNA as a surrogate marker of daily protein intake (R=0.51; R2=0.29; R2 adjusted=0.23; p<0.001).
ConclusionControlled protein intake is one of the cornerstones of treatment in CKD patients. A low protein intake in patients with CKD stages 3 and 4–5 was associated with loss of muscle mass in the advanced-CKD unit. The loss of muscle mass appears as an early indicator of nutritional comprised. Factors such, elderly age and loss of eGFR, showed lower protein intake and were associated with muscle loss, especially in women. Further longitudinal studies are required to evaluate the contribution of different protein intakes to uremic symptoms, nutritional status, body composition and CKD progression.
El control de la ingesta proteica ha mostrado efectos beneficiosos preservando la función renal y el estado nutricional en pacientes con enfermedad renal crónica (ERC). El objetivo del estudio fue analizar la ingesta habitual de proteína, y su potencial contribución en la composición corporal en los pacientes con ERC estadios 3-5.
MétodoEstudio observacional transversal en 134 pacientes con ERC estadios 3-5 (media e-TFG: 19,4±8,7ml/min/1,73m2; varones: 68,7% y etiología primaria de la ERC, diabetes mellitus: 35,8%). Se evaluaron parámetros demográficos, clínicos y nutricionales. La aparición de nitrógeno proteico normalizado (nPNA) se utilizó como marcador sustituto de la ingesta proteica. La muestra fue clasificada según el nPNA en 3 grupos (Gn): G1: < 0,8g/kg/día; G2: 0,8-1g/kg/día y G3:≥1g/kg/día. Valoración nutricional por la escala de malnutrición-inflamación (MIS), medidas antropométricas y parámetros de laboratorio. Análisis de composición corporal y del patrón de hidratación mediante bioimpedancia eléctrica (BIVA-101®, RJL System). Análisis estadístico por SPSS® v.20.
ResultadosGlobalmente los valores medios de nPNA fueron 0,91±0,23g proteína/kg peso corporal/día, y tan solo el 32,1% tenían una ingesta proteica <0,8g de proteína/kg peso corporal/día. El 65,5% de los pacientes con ERC estaban en estadios 4 y 5. La prevalencia de síndrome de desgaste proteico-energético (SDP) medido por MIS era del 15%. Analizando las diferencias con el nPNA entre los grupos, el peso corporal, el índice de masa corporal y el pliegue tricipital (PCT), eran significativamente mayores en el grupo con nPNA≥1g/kg peso corporal/día (G3), mientras que se encontró relación inversa significativa con los porcentajes de la masa celular (MC%), de la masa magra (MMagra%), de la masa muscular (MM%) y del ángulo de fase (AF) en el grupo con menor nPNA (G1). El análisis del género entre los sujetos mostró diferencias significativas con el peso corporal, MMagra%, PCT y la circunferencia muscular del brazo (CMB%). El análisis de regresión lineal demostró que la resistencia MC%, MM% y la albúmina sérica eran predictores significativos del nPNA como marcador de la ingesta proteica habitual (R=0,51; R2=0,29; R2 ajustado=0,23; p<0,001).
ConclusiónEl control de la ingesta proteica es uno de los pilares del tratamiento en los pacientes con ERC. La dieta hipoproteica en pacientes con ERC estadios 3-5 se asoció con una pérdida de la masa muscular en la unidad de ERC avanzada. La pérdida de masa muscular aparece como un indicador temprano de compromiso nutricional. La edad avanzada y la pérdida de TFG-e se asociaron con menor ingesta proteica y pérdida de masa muscular asociada, especialmente en mujeres. Nuevos estudios longitudinales, son necesarios para evaluar la contribución de la ingesta de proteínas en los síntomas urémicos, el estado nutricional, la composición corporal y la progresión de la ERC.
Dietary protein intake is a pivotal issue in the progression and treatment of the chronic kidney disease (CKD) patients. The early primary care on moderate-to-advanced CKD stages of well-known causative factors such as reduction of accumulation of nitrogenous wastes, metabolic derangements and protein-energy wasting (PEW), have showed a central role to slow onset of dialysis1,2 and mortality rates3 in CKD population.
Guidelines on CKD4,5 and Nutrition,6,7 recommend two tentative nutritional approaches for managing dietary protein intake in CKD stages 4,5. A low-protein diet (LPD), consists of 0.6–0.8g/kg BW/day or a very low-protein diet (VLPD), providing a protein intake between 0.3 and 0.4g/kg BW/day supplemented with essential aminoacids (EAA) and ketoanalogues (KA). Both therapeutic strategies preserve renal function in well-nourished patients8–10 whereas, the ideal recommendation of dietary protein restriction on the progression of CKD, and its potential consequences on nutritional status and body composition still unclear.
PEW is frequently associated with higher risk of morbidity and mortality.3 Lack of appetite and uremic anorexia interferes on nutritional status and body composition measures in CKD patients. Bioelectrical impedance (BIA), is a non-invasive method for the assessment of body composition and conditions associated with expanded extracellular water (ECW).11 Previous studies12,13 reported that BIA-derived measures may be important in glomerular filtration rate (GFR) prediction and several studies14,15 demonstrated that the inclusion of muscle mass improved GFR estimations. Dumler et al.,16 showed that CKD patients with stable renal function following a daily dietary protein intake (0.6–0.8g/kg normalized body weight/day), had no loss of body cell mass (BCM) or fat-free mass (FFM) over a 9-month period measured by BIA. Thus, assessment and follow-up by nutritional indicators and body composition measures could be useful to early diagnosis of being wasted. These results push up to study whether the body composition in CKD patients stages 3–5 according to their daily dietary protein intake might predispose to muscle wasting because of dietary restrictions and the catabolic effects of uremia. There is little evidence with regard to the dietary protein intake restriction on body composition in moderate-advanced CKD. This study aimed to analyze usual dietary protein intake and its potential contribution on body composition in CKD patients on stages 3–5.
Patients and methodsStudy designThis cross-sectional study was carried out at Hospital Universitario de La Princesa, Madrid (Spain) in 134 CKD patients on stages 3–5. Eligible participants were CKD adults (≥18 years) stabilized for at least (minimum) of 3 months before enrolment. Patients with amputated limbs, clinically evident active infection, liver disease, autoimmune diseases, or malignancies were excluded to avoid the possible effects of these comorbid conditions on inflammatory markers and on body composition status. Informed consent was obtained from every participant. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethic Committee of Hospital de la Princesa (code number: 2849).
Clinical assessmentDemographic, clinical and nutritional data were obtained from the medical history of each patient. The monographic nutrition CKD consultation at advanced-CKD unit, was usually recommended 0.8g/kg BW/day of protein intake and dietary salt-restriction according to the guidelines for CKD patients.4 Nutritional counseling and follow-up is carried out every three months for ensuring adherence to nutritional recommendations. Serial nPna measures and body composition analysis at each three-month visit, are routinely performed in order to check compliance with protein prescription. LPD supplemented with KA, were not currently used for managing CKD at the advanced CKD-unit. Glomerular filtration rate was estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation for the estimated glomerular filtration rate (e-GFR).17
Normalized protein nitrogen appearance (nPna) as a surrogate indicator of dietary protein intake according to the equation proposed by the Kidney Disease Outcomes Quality Initiative clinical practice guidelines18 was calculated as follows:
The sample was classified into three nPna groups (Gn) according the daily mean of the protein intake (to mean of daily protein intake): (G1): nPna<0.8g/kg/day; (G2): nPna 0.8–1g/kg/day and, (G3): nPna≥1g/kg/day.
Nutritional assessmentNutritional status was assessed by the malnutrition-inflammation score (MIS) questionnaire,19 including six different components: five subjective assessments (concerning the patient's medical history and physical examination), and three objective assessments [s-albumin, total binding iron capacity and body mass index (BMI)]. In agreement with other studies,20 PEW was defined as a MIS score ≥5.
Anthropometric measurementsBody weight (BW), BMI, standard body weight (SBW), triceps skinfold thickness (TSF), and mid-arm muscle circumference (MAMC) were recorded as anthropometric measures. The BMI as dry weight in kilograms divided by the square of height in meters was calculated. TSF was measured with a Lange Skin Calipers (Holtain Caliper, Crymch, Dyfed, UK), using standard techniques. MAMC was estimated as follows: MAMC (cm)=mid-arm circumference (cm)−0.314×TSF (mm). All anthropometric measurements were done in duplicate by the same investigator, and the mean values were taken for the analysis.
Handgrip strength was performed on the dominant arm or non-fistula side, using a manual dynamometer (Baseline® Hydraulic Hand Dynamometer – 12-0240; Irvington, NY, USA) device. All anthropometric measures were repeated three times and the mean value was taken for the analysis.
Body composition analysisMonofrequency BIA was determined on the non-dominant side of the body, in the post-absorptive state, injecting 800μA alternating sinusoidal current with a standard tetrapolar technique (BIA 101 Impedance Analyzer; Akern, Firenze, Italy). BIA was performed at fasting state in the supine position21,22 with disposable electrodes (BiatrodesTM 100'S; Akern). Body composition analysis by gender, age, BW and height was individually measured at 50kHz, which resistance and reactance were obtained.
The BIA-derived variables [(exchange Na/K, percentage of total body water (TBW%), extracellular body water (ECW%), intracellular body water (ICW%), body cell mass (BCM%), fat mass (FM%), muscle mass (MM%), fat-free mass (FFM%) and values of phase angle (PA)], were estimated by BIA® software. This method was previously validated in CKD11 and dialysis patients.23
Laboratory parametersEarly morning blood samples were drawn from every patient in a 12-h overnight fasting conditions. Serum albumin (s-albumin) using bromocresol green method was measured using an automated analyzer (Abbot, Aeroset®, Diamond Diagnosis, Holliston, MA).24 Variation coefficients were lower than 2%. CRP (no-high sesnsitivity), was measured by immunoturbidimetry (Roche/Hitachi 904®/Model P: ACN 218, Roche Diagnostics, Basel, Switzerland). S-CRP was logarithmically transformed to normality using the natural log (Ln s-CRP).
Statistical analysisThe statistical analyses were carried out using the SPSS v.20 software and the results expressed as the arithmetic mean and standard deviation. Correlation between continuous variables by Pearson test was calculated. The normality and homoscedasticity of the data were verified by the Kolmogorov–Smirnov and Shapiro–Wilk test, respectively. The analysis of variance (ANOVA) followed by the Bonferroni and Tukey post-hoc test was used when the variables showed a normal distribution and Welch and Brown–Forsythe when the variables were non-parametric. Analysis of covariance (ANCOVA) was used to test extent variations of gender as a covariate. Linear regression analysis was used to test the effect of nPna on other variables. The level of significance was set at p<0.05.
ResultsDemographic characteristicsA total of 134 CKD patients participated in the study. The aged population was 70.9±13.11 years, 68.7% were men and the primary etiology of CKD was diabetes mellitus (35.8%) (Table 1). Most of the patients were in CKD stage 3 (34.5%) and stage 4 (56.5%), with mean nPna values of 0.84±0.21 and 0.93±0.21g/kg/day, respectively (p=0.033).
Demographic and laboratory data in 134 CKD patients stages, 3–5 according to daily dietary protein intake.#.
| Variables | Global | G1: nPna <0.8g/kg/day | G2: nPna 0.8–1g/kg/day | G3: nPna ≥1g/kg/day | p-value* |
|---|---|---|---|---|---|
| n | 134 | 43 | 53 | 38 | |
| nPna (g/kg BW/day) | 0.91±0.23 | 0.65±0.08 | 0.89±0.21 | 1.16±0.16 | <0.001 |
| Age (years) | 71±13.11 | 73.58±12.58 | 70.22±12.27 | 69.21±14.79 | 0.29 |
| Men, n (%) | 92 (68.7) | 25 (17.9) | 40 (29.9) | 27 (20.1) | <0.001 |
| Diabetes, n (%) | 48 (36.1) | 24 (17.9) | 28 (20.9) | 3 (12.7) | 0.73 |
| e-GFR (mL/min) | 19.4±8.7 | 16.34±4.95 | 17.43±5.73 | 17.73±5.53 | 0.16 |
| Hemoglobin (g/L) | 12.25±1.3 | 11.86±1.14 | 12.19±1.28 | 12.50±1.25 | 0.076 |
| s-Albumin (g/dL) | 4.18±0.42 | 4.04±0.46 | 4.20±0.44 | 4.11±0.42 | 0.015b |
| Total Lymphocytes count (×103/mm3) | 2026.4±1133.4 | 1897.5±881.19 | 2142±1529 | 2016.87±744.23 | 0.44 |
| s-Creatinine (mg/dL) | 3.27±1.21 | 3.39±1.08 | 3.54±1.17 | 3.42±1.16 | 0.94 |
| Ln s-CRP | −0.84±1.13 | −0.75±1.09 | −0.87±0.99 | −1.1±1.1 | 0.38 |
| Proteinuria (mg/dL) | 58.4±69.7 | 82.34±72.1 | 49.5±63.5 | 44.70±70.3 | 0.032 |
| PEW,†n (%) | 20 (15) | 7 (5.2) | 8 (6) | 5 (3.7) | 0.33 |
e-GFR, estimated glomerular filtration rate measured by CKD-EPI equation; Ln s-CRP, natural logarithmic of C-reactive protein; nPna, normalized protein nitrogen appearance. PEW, protein-energy wasting.
*p-values are based on Chi-square tests or ANOVA-tests according to cut-off points of normalized protein nitrogen appearance (nPna).
#Daily protein intake measured by nPna were defined in different groups as follows: G1: nPna<0.8g/kg BW/day; G2: nPna 0.8–1g/kgBW/day and; G3: nPna≥1g/kg BW/day. a,b,cp-values within the means of nPna bearing different letters were significantly different; ap<0.05, bp<0.01, cp<0.001 (Brown–Forsythe test for equal means).
†PEW was defined by MIS score ≥5 points.
nPna was positively and significantly associated with e-GFR (r=0.19; p<0.05), BCM% (r=0.32; p<0.001), MM% (r=0.42; p<0.001) and s-albumin (r=0.26; p<0.01), whilst inverse correlation was observed between nPna and exchange Na/K (r=−0.18; p<0.05). Not significant correlation between nPna and Ln s-CRP was found.
Patient characteristics according to dietary protein intakeTable 1 shows laboratory data according to nPna groups of daily protein intake. Of the 134 CKD patients, 43 patients (32.1%) had nPna<0.8g/kg BW/day (G1), 53 patients (39.2%) with nPna of 0.8–1g/kg BW/day (G2) and 38 patients (28.3%) had a nPna>1g/kg BW/day (G3). Patients with lower nPna (G1), were older and showed also significantly higher proteinuria in comparison with mean values of nPna>0.8g/kg BW/day. Mean values of s-albumin showed significantly differences between nPna groups (G1, G2, G3), being highlighted mean values of albumin level >4g/dL in the three groups (p=0.015). Ln s-CRP tended in a non-significant manner to be higher levels with nPna≥1g/kg BW/day. The prevalence of PEW was 15% in this study.
Table 2 shows anthropometric, nutritional and body composition data according to nPna groups of daily protein intake. Anthropometric measures as BW, BMI and TSF were significantly higher within nPna≥1g/kg/day group. MAMC%, tended significantly to be decreased across nPna groups but, being within normal range. As regards the BIA-derived hydration parameters, patients with low protein intake had higher values of exchange Na/K (p>0.05), and ECW% (p<0.01), whereas TBW% (p<0.05), ICW% (p<0.01) and s-albumin (p<0.015) were also significantly lower with nPna<0.8g/kg BW/day (G1). Low protein intake (G1) showed significantly lower FFM%, MM% and PA values, but not significant differences with handgrip strength (kg) were found. Interestingly, MM% was higher with nPna values ≥0.8g/kg BW/day.
Anthropometric, nutritional and body composition data in 134 CKD patients stages, 3–5 according to daily dietary protein intake.#.
| Variables | Global | G1: nPna <0.8g/kg/day | G2: nPna 0.8–1g/kg/day | G3: nPna ≥1g/kg/day | p-value* |
|---|---|---|---|---|---|
| Body weight (kg) | 74.41±15.13 | 77.78±15.06 | 76.32±13.99 | 69.32±15.96 | 0.037a |
| Standard Body Weight (kg) | 68.08±8.26 | 67.77±7.28 | 70.01±8.24 | 68.62±10.18 | 0.301 |
| BMI (kg/m2) | 27.19±4.99 | 28.42±5.23 | 27.40±4.28 | 25.56±4.67 | 0.012b |
| TSF (mm) | 17.84±9.35 | 20.02±11.14 | 16.79±7.81 | 14.75±7.09 | 0.016b |
| MAMC (cm) | 27.9±4.3 | 28.96±4.86 | 28.14±3.62 | 26.58±4.09 | 0.041a |
| MAMC (%) | 130.2±22.1 | 136.1±27.44 | 130.97±18.49 | 122.59±18.1 | 0.024a |
| Handgrip strength right (kg) | 25.4±9.4 | 24.1±9.3 | 26.9±9.35 | 25.1±9.58 | 0.34 |
| Handgrip strength left (kg) | 23.8±9.1 | 22.3±8.69 | 24.8±9.32 | 24.2±9.19 | 0.41 |
| Exchange Na/K | 1.46±0.53 | 1.58±0.66 | 1.47±0.52 | 1.45±0.42 | 0.463 |
| Body cell mas (%) | 37.3±9.34 | 34.1±8.17 | 37.7±9.8 | 40.5±1.45 | 0.008b |
| Total body water (%) | 54.4±7.2 | 52.90±8.58 | 55.77±7 | 55.63±4.91 | 0.037 |
| ECW (%) | 56.45±8.72 | 59.86±8.57 | 55.99±8.57 | 55.10±8.51 | 0.007b |
| ICW (%) | 43.51±8.73 | 40.13±8.57 | 44±8.57 | 44.79±8.6 | 0.008b |
| Fat mass (%) | 31±9.5 | 33.46±10.91 | 29.50±8.96 | 28.80±7.89 | 0.012 |
| Fat-free mass (%) | 68.9±9.5 | 66.31±11.03 | 70.49±8.96 | 71.19±7 | 0.009b |
| Muscle mass (%) | 33.78±7.71 | 29.68±5.06 | 34.82±8.29 | 55.77±7 | <0.001c |
| Phase angle (°) | 4.17±1.23 | 3.77±1.14 | 4.26±1.25 | 4.27±1.24 | 0.033 |
BMI, body mass index; ECW, extracellular water; FM, fat mass; FFM, fat free mass; ICW, intracellular water; MAMC, middle-arm muscle circumference; MIS, Malnutrition-Inflammation Score; TBW, total body water; TSF, triceps skinfold thickness.
*p-values are based on Chi-square tests or ANOVA-tests according to cut-off points of normalized protein nitrogen appearance (nPna).
#Daily protein intake measured by nPna were defined in different groups as follows: G1: nPna < 0. 8g/kg BW/day; G2: nPna 0.8–1g/kg BW/day and; G3: nPna ≥ 1g/kg BW/day. a,b,cp-values within the means of nPna bearing different letters were significantly different; ap<0.05, bp<0.01, cp<0.001 (Brown–Forsythe test for equal means).
In a sub-analysis data, robust test of equal means showed significant differences with BCM%, ECW%, FFM%, MM% and s-albumin in the sample (at least, p<0.01). Gender inter-subjects’ analysis showed significant differences with BW, FFM%, TSF and MAMC%.
Protein intake as a predictor of nutritional and body composition status in chronic kidney disease patientsIn the linear regression analysis adjusted by gender, age and BW (Table 3), parameters as resistance, BCM%, MM%, and s-albumin were significantly predictors of nPna as a surrogate marker of daily protein intake (R=0.51; R2=0.29; R2 adjusted: 0.23; p<0.001).
Normalized nitrogen protein appearance in a linear regression analysis as likely predictor in CKD stages 3–5 patients.#.
| Variable | Coefficient | 95%CI | p-value |
|---|---|---|---|
| Resistance | 0.276 | 0.00 to 0.001 | 0.01 |
| Exchange Na/K | 0.445 | −0.136 to 0.522 | 0.24 |
| Body cell mass (%) | −1.996 | −0.008 to −0.092 | 0.019 |
| Fat-free mass (%) | 0.574 | −0.026 to 0.054 | 0.485 |
| Muscle mass (%) | 2.724 | 0.028 to 0.139 | 0.004 |
| Fat mass (%) | 2.161 | 0.000 to 0.107 | 0.050 |
| s-Albumin (g/dL) | 0.203 | 0.012 to 0.209 | 0.028 |
| Constant | −8.63 to 0.97 | 0.117 |
#Normalized nitrogen protein appearance as a dependent variable in a linear regression model adjusted by gender, age and body weight. 95%CI, confidence interval. R=0.53; R2=0.29; R2 adjusted=0.23.
The results of this study revealed that low dietary protein intake in CKD patients on stages 3, 4–5, was associated with loss of muscle mass even in patients receiving dietary counseling and nutritional follow-up at the advanced-CKD unit. In this study, older ages and lower e-GFR in CKD patients showed lower protein intake and loss of muscle mass, especially in women.
nPna an indirect measure of protein intake was showed as a strong and independent predictor of morbidity and mortality in CKD patients25 and dialysis patients.26 Clinical practice guideline for CKD patients,4–7 defined as those with e-GFR<30mL/min/1.73m2, have recommended reducing dietary protein intake to 0.8g/kg/day in adults both with and without diabetes to slow disease progression, and achieve/maintain adequate s-albumin concentration and nutritional status. In the current study, overall nPna values were slightly increased, but only 57% of the eligible CKD patients correctly achieved the low protein intake reduction. Patients with CKD on stage 3, had mean nPna values closed to current protein intake recommendation, whilst advanced CKD on stages 4–5, showed higher daily protein intake. In this study, diet adherence of usual nutritional recommendation was low, being observed a 67.9% of CKD patients with nPna≥0.8g/kg BW/day. However, a dietary protein intake <0.8g/kg BW/day (G1), showed significantly depletion of MAMC, BCM%, FFM% and PA in a similar way compared with one another nPna categories. These findings are of importance because the loss of muscle mass and wasting has been demonstrated to be associated with poor outcomes in CKD patients.27,28
PEW is common in CKD patients and is associated with a subsequent increased risk of mortality.3 Dietary protein and energy intake may be masked in patients with advanced CKD due to uremic anorexia, inter-current illness, systemic inflammation, fluid overload and/or excessive dietary protein restriction. S-albumin concentration and BMI are the most often used indicators of nutritional assessment in daily clinical practice29,30 However, both are influenced by several non-nutritional factors, including proteinuria, fluid overload and inflammation statuses.31,32 In the current study, PEW was assessed by MIS, as a nutritional screening tool previously validated in CKD and dialysis patients together with commonly used nutritional-inflammatory markers. Overall, only 15% of CKD patients were wasted. In accordance with previous studies,33,34 the prevalence of PEW in the study was low compared with others.35,36 Furthermore, despite of low e-GFR (mean 19.4±8.7mL/min/1.73m2), a common used marker of protein turnover such as s-albumin was ≥4g/dL in all nPna groups. Therefore, a routine nutritional screening (i.e. MIS) could decreased the contribution of each indicator by combining s-albumin and BMI. In fact, this study found significantly positive correlation between nPna with e-GFR, BCM%, MM% and s-albumin, but not Ln s-CRP and BMI. These findings suggest that a multifactorial approach in CKD patients is mandatory.
Additional methods such as BIA and anthropometric measures may be also clinically useful to assess nutritional status. Body composition analysis in patients with CKD are characterized by high content of ECW associated with sodium retention and a decreased of BCM, both related with well-known causes as inflammation, fluid overload and wasting.22,31,32 In this study, exchange Na/K and ECW% were significantly higher as protein intake was <0.8g of protein/kg BW/day. Additionally, BIA-derived parameters such as FFM% and MM% were also diminished. Linear regression analysis showed that BIA-measures as resistance, MM% and s-albumin were independent predictors of nPna in the study population, but an inverse relation was found with BCM%. These findings suggest that a high protein intake could worsen renal function and, consequently the homeostasis of internal environment. Nevertheless, parameters such as exchange Na/K, FFM% and FM% were not found significant predictors of nPna in the linear regression analysis. Thus, in addition to common used nutritional markers, data suggest that body composition parameters should be taken into account in the nutritional monitoring, in patients with CKD. In this clinical setting, a “tailored diet prescription” considering gender-aged and body composition differences is required.
Prescription of a KA/EAA supplemented VLPD may be considered in well-nourished not-inflamed CKD patients aimed to preserve the renal function and metabolic disturbances related to uremic milieu. However, in wasted patients with alterations of fluid status and body composition parameters may be further considered dietary protein intake definition. There are potential limitations to be taking into account in this study. The sample size of this study due to its nature as a single-center cross-sectional study may reduce the generalizability of the findings. First, there are fluctuations in nPna from day to day caused by changes in daily protein intake or endogenous protein catabolism related with e-GFR value in CKD patients. Monitoring sequentially the body composition measures (i.e. muscle mass), could provide early nutritional interventions. Second, for nPna to accurately estimate protein intake, the patient's protein metabolism should be at equilibrium or nearly so at the time of measurement. This condition is not always met, particularly in CKD patients with many comorbidities, inflammation or acute disease states. In this study, eligible CKD patients were stabilized for at least 3 months before enrolment. Third, nPna could be masked usual dietary protein intake in wasted-inflamed patients. Assessment of nutritional-inflammatory status by validated nutritional tools, could early diagnose inadequate food intake and nutritional related-problems (i.e. PEW) in CKD patients.
In conclusion, controlled protein intake is one of the mainstays of CKD patients. A low protein intake in patients with CKD stages 3, 4–5, was associated with muscle wasting, even in patients receiving dietary advice and nutritional follow-up in the advanced-CKD unit. The loss of muscle mass appears as an early indicator of being wasting. Factors such as advanced age and loss of eGFR, showed lower protein intake and were associated with muscle loss, especially in women. In addition, of standard medical care, assessment of nutritional status and body composition should be included to prevent, diagnose and/or treat PEW.
Further longitudinal studies are required for evaluating the contribution of different protein intake in uremic symptoms, nutritional status, body composition and CKD progression.
Authors’ contributionsGB designed the study, collected the data, analyzed and interpreted the results, and drafted the manuscript. AN collected the data and drafted the manuscript. MR, analyzed, interpreted the results, and drafted the manuscript. YC analyzed and interpreted the statistical results. JST analyzed, interpreted the results, and drafted the manuscript. All of the authors read and approved the final version of manuscript.
DeclarationsNone.
Conflict of interestsThe authors declare that they have no conflict of interests.
We are grateful to patients for their voluntary participation in this study.