Two cases of children diagnosed with renal tubular acidosis (RTA) associated with autoimmune hypothyroidism are presented.
Case 1 developed an intestinal ileus at the age of five in the context of a respiratory problem. The tests performed confirmed metabolic acidosis, hyperchloraemia, hypokalaemia and nephrocalcinosis. Case 2 was diagnosed with hypothyroidism at the age of 11, and with RTA two years later.
In both patients, the diagnosis of RTA was verified when decreased maximum urinary pCO2 was found. In case 2, a proximal bicarbonate leak (type 3 RTA) was also confirmed. This was the first case to be published on the topic.
The causes of RTA in patients with hypothyroidism are reviewed. The deleterious effect on the kidneys may be due to the absence of thyroid hormone and/or autoantibodies in the cases of autoimmune hypothyroidism.
Se presentan dos casos en edad pediátrica diagnosticados de acidosis tubular renal (ATR) asociada a hipotiroidismo de causa autoinmune.
El caso 1 desarrolló un íleo intestinal a los 5 años de edad en el seno de un problema respiratorio. En los exámenes realizados se constató acidosis metabólica, hipercloremia, hipopotasemia y nefrocalcinosis. El caso 2 fue diagnosticado de hipotiroidismo a los 11 años de edad y 2 años después, de ATR.
En ambos pacientes, se comprobó el diagnóstico de ATR al observarse una pCO2 urinaria máxima reducida. En el caso 2 se constató, además, una fuga proximal de bicarbonato (ATR tipo 3), que constituye el primer caso publicado sobre el tema.
Se revisan las causas de ATR en pacientes con hipotiroidismo. El efecto deletéreo sobre el riñón puede ser debido a la propia ausencia de hormona tiroidea y/o a los autoanticuerpos en los casos de hipotiroidismo autoinmune.
The term renal tubular acidosis (RTA) is applied to a group of transport defects in the reabsorption of bicarbonate in the proximal tubule (proximal or type 2 RTA), the excretion of hydrogen ions (H+) in the collecting tubule (RTA type 1 and 4) or both (RTA type 3).1 It is characterised by the presence of persistent hyperchloraemic metabolic acidosis with a normal anion gap.
Primary forms are more common in infants and children.2 Distal RTA is the most common variant. In recent years, molecular biology techniques have identified the genetic factors involved in inadequate urinary excretion of H+ and ammonium in patients with distal RTA.3 This defect can be diagnosed by NH4Cl loading (rich in H+) or by determining maximum urinary pCO2 (UpCO2).4 Clinically, distal RTA is characterised by the presence of polyuria, polydipsia, nephrocalcinosis, hypokalaemia, persistently alkaline urine, hypercalciuria, hypocitraturia, and a defect in renal concentrating capacity, with a tendency towards dehydration.5
Secondary forms of distal RTA, more common in adults, are associated with the administration of drugs or toxins, or with systemic or immunological diseases. In children, secondary causes are less common, and have been described in cases of Sjögren's syndrome6 or systemic lupus erythematosus,7 or after oral intake of some medications.
Few cases of distal RTA associated with classic hypothyroidism or with autoimmune thyroid disease have been reported. Even fewer cases have been reported in children. We report the cases of two girls with acquired hypothyroidism associated with secondary distal RTA. The association between hypothyroidism and type 3 RTA in one of the patients is the first case reported in the literature.
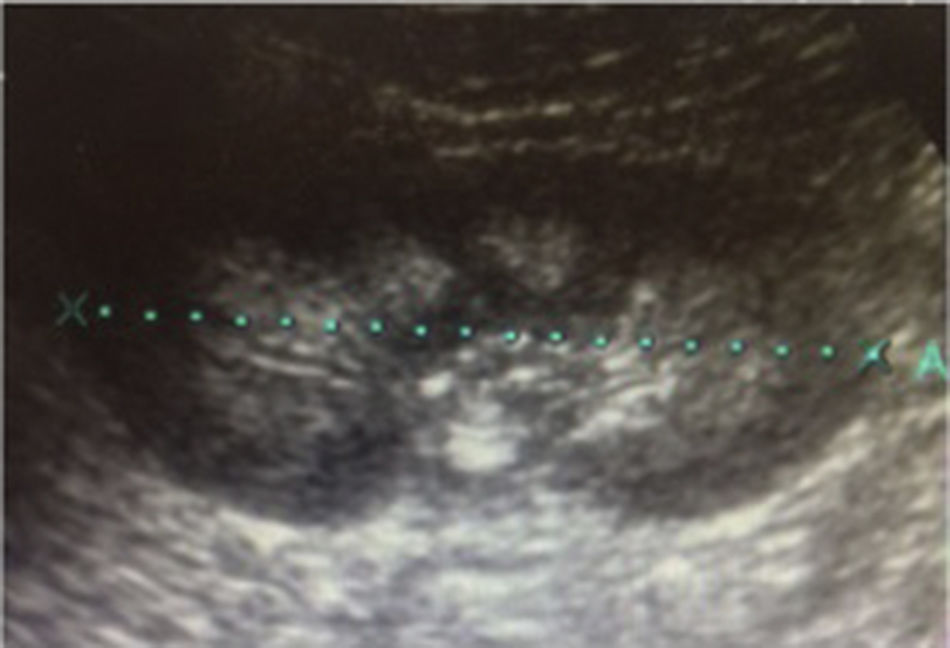
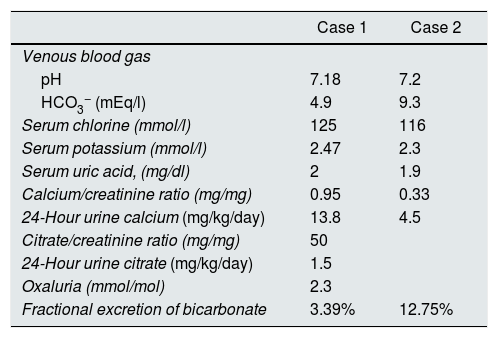
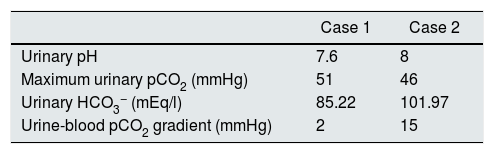
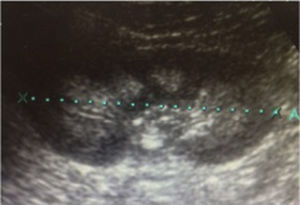
Case 1A girl aged 5 years and 7 months, with a history of abdominal distension from the first months of life, together with arrested weight gain and growth from the age of 2 (below the 3rd percentile). She was admitted due to a 3-day history of fever of 39°C, greenish rhinorrhoea, productive cough and pharyngeal pain, accompanied by progressive respiratory difficulty, polypnoea and abdominal distension. On admission, the physical examination found severe dehydration, dry oral mucosa, tachycardia, signs of pulmonary consolidation in the right hemithorax, and distended abdomen with decreased peristalsis. A chest X-ray confirmed the diagnosis of pneumonia, and an abdominal X-ray was compatible with metabolic ileus. Laboratory test findings included metabolic acidosis, hyperchloraemia and hypokalaemia, which persisted despite restoration of the fluid balance (Table 1). A urine test found hypercalciuria and hypocitraturia (Table 1) and the kidney ultrasound showed grade 2 nephrocalcinosis (Fig. 1). An acetazolamide- and sodium bicarbonate-loading test was performed, obtaining a maximum urinary pCO2 of 47mmHg at 60min (urine-blood pCO2 gradient: 15mmHg) (Table 2). The audiometry test was normal.
Blood and urine biochemical tests.
| Case 1 | Case 2 | |
|---|---|---|
| Venous blood gas | ||
| pH | 7.18 | 7.2 |
| HCO3− (mEq/l) | 4.9 | 9.3 |
| Serum chlorine (mmol/l) | 125 | 116 |
| Serum potassium (mmol/l) | 2.47 | 2.3 |
| Serum uric acid, (mg/dl) | 2 | 1.9 |
| Calcium/creatinine ratio (mg/mg) | 0.95 | 0.33 |
| 24-Hour urine calcium (mg/kg/day) | 13.8 | 4.5 |
| Citrate/creatinine ratio (mg/mg) | 50 | |
| 24-Hour urine citrate (mg/kg/day) | 1.5 | |
| Oxaluria (mmol/mol) | 2.3 | |
| Fractional excretion of bicarbonate | 3.39% | 12.75% |
After reaching a diagnosis of distal RTA, treatment with potassium citrate solution was initiated (4mEq/kg/day), with no improvement in weight and height, and persistence of asthenia, adynamia and somnolence. The thyroid function test showed thyroid-stimulating hormone (TSH) levels of more than 150mU/l, T3 levels of 0.19ng/ml and undetectable T4. Blood samples were positive for antithyroglobulin (181.6IU/ml) and antiperoxidase (373.2IU/ml) antibodies. Levothyroxine was started, and the patient's weight and height improved. Now, at 11 years of age, her weight is 29kg (p8%) and her height 133cm (p6%); urinary acidification defect persists (maximum urinary pCO2 of 49mmHg).
No molecular alterations were observed in ATP6V0A4, ATP6V1B1 and SLC4A1 genes analysed by targeted exon and exon-intron transition sequencing.
Case 2This was a 13-year-old girl, with no significant family history, with arrested weight gain and growth from the age of 7. Since then, she had presented 4 episodes of limb paralysis and generalised weakness, with craniocervical instability and bilateral genu valgum. At age 11 she was diagnosed with hypothyroidism, and started treatment with levothyroxine. She was subsequently referred to the hospital for a hypokalaemia study. The results of the blood and urine panels and acidification test are shown in Tables 1 and 2. Along with the distal acidification defect, a bicarbonate leak in the proximal tubule, compatible with type 3 RTA was observed (Table 1). Kidney ultrasound showed bilateral grade 2 nephrocalcinosis. The audiometry assessment was normal. Blood samples were positive for antithyroglobulin (246.8IU/ml) and antiperoxidase (92.6IU/ml) antibodies. One year after the diagnosis of RTA, the patient required higher doses of citrate, and the urinary acidification defect (maximum urinary pCO2 of 53mmHg) persists. Now, at 14 years of age, she weighs 40kg (p5%) and her height is 138cm (p0%).
DiscussionThyroid hormones influence kidney development, kidney structure, renal haemodynamics, glomerular filtration rate, the function of many transport systems along the nephron, in particular those related to the management of sodium, acid–base balance and renal concentrating capacity. These effects of the thyroid hormone are in part due to direct renal actions, and in part to cardiovascular and systemic haemodynamic effects that influence kidney function.8
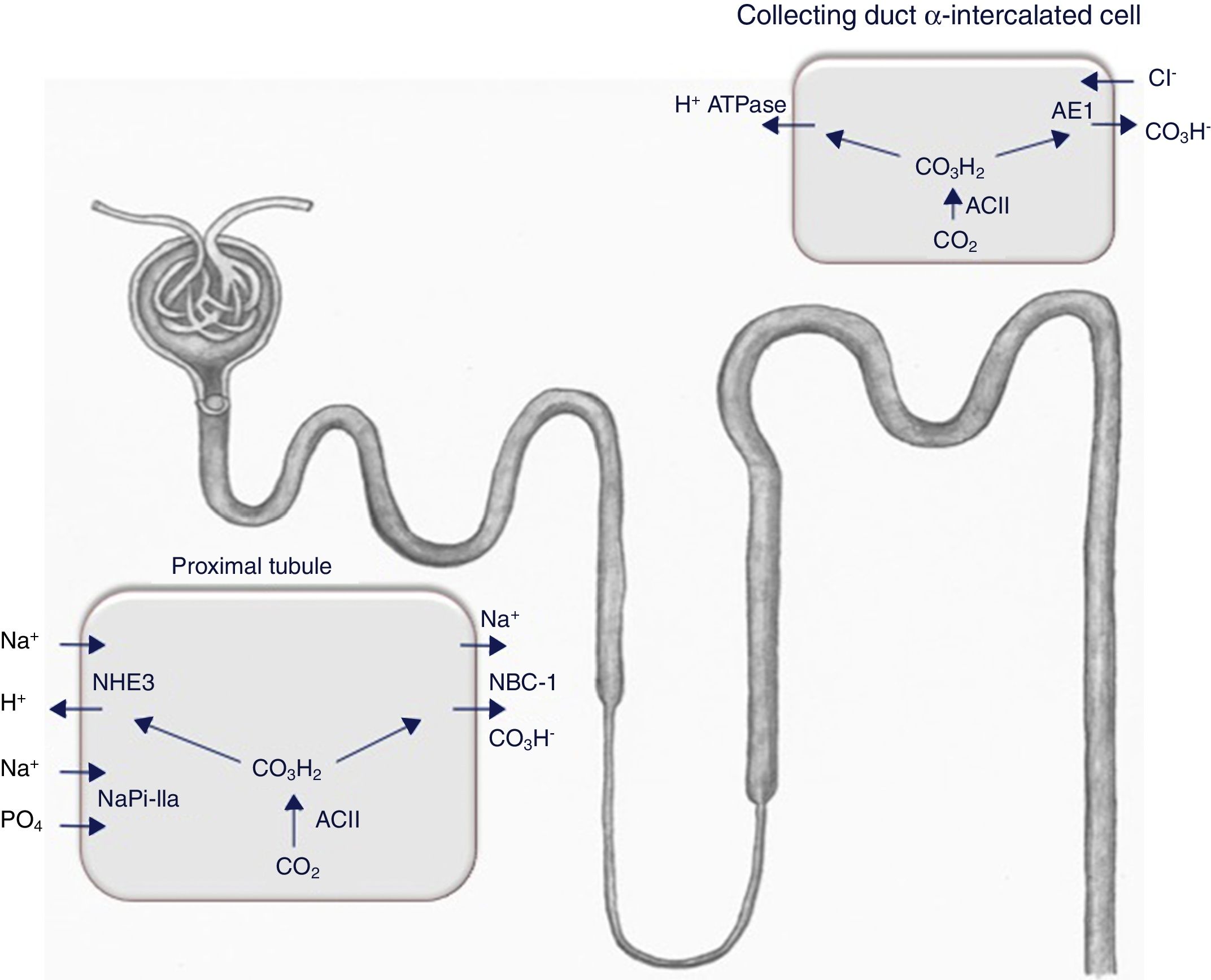
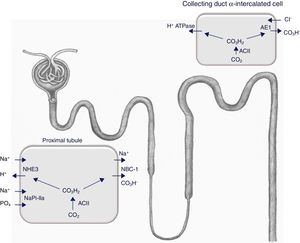
Thyroid hormones directly influence the expression and/or activity of a number of ion channels and transporters. Pioneering micropuncture studies in hypothyroid rats have shown a reduction in the renal glomerular filtration rate (GFR) and an increase in the urinary excretion of sodium and water.9 A subsequent study reported that the thyroid hormone regulates the activity of the proximal tubular type 3 Na+/H+ exchanger (NHE3)10 (Fig. 2), an effect that is due to direct binding of the hormone with the promoter region of the encoding gene.11
The expression of the Na+/H+ exchanger (NHE3), the Na+-phosphate cotransporter NaPi-IIa, and the B2 subunit of the vacuolar H+-ATPase (not shown here) is reduced in the brush-border membrane of the proximal tubule in hypothyroid rats. This is accompanied by a lower abundance of the Na+/HCO3− cotransporter (NBCe1) and a higher expression of the acid-secretory type A intercalated cell-specific Cl−/HCO3− exchanger (AE1) in the connecting tubule and cortical collecting duct. NBCe1 expression and the number of α-intercalated cells increased in hypothyroid rats during metabolic acidosis.12 ACII: carbonic anhydrase II.
A study published in 2007 confirmed the activity of several transporters involved in sodium and acid–base balance in rats rendered hypothyroid by the administration of methimazole.12 The results obtained are summarised in the footer of Fig. 2. Basically, the activity of several proximal tubule transporters is reduced, which results in a loss of sodium and a reduction in the elimination of hydrogen ions at this level, while expression of the Cl−/HCO3− (AE1) exchanger specific to α-intercalated cells of the connecting tubule and cortical collecting duct increases.12 This shows, therefore, that thyroid hormone deficiency is associated with a defect in the renal handling of sodium and of the acid–base balance, which is mainly located in the proximal tubule and is compensated by the distal nephron. Consequently, on a physiological level, the thyroid hormone modulates the renal response to acid overload and alters the expression of several transporters that are key in the maintenance of the acid–base balance.
This begs the question of why significant proximal sodium loss does not lead to reduced levels of sodium in hypothyroidism. This was studied in transgenic mice deficient in the Na+/H+ (NHE3) exchanger13 and in adults with mutations in the encoding gene.14 In these cases, loss of NHE3 was compensated by a reduction in GFR caused by adenosine-induced vasoconstriction of the afferent arteriole. This is one of the reasons why GFR is reduced in hypothyroid patients.15,16
A study published in the 1970s showed that 2 of 5 adults with primary hypothyroidism without metabolic acidosis and with no clinical evidence of autoimmune disease were unable to lower their urine pH appropriately after short duration acid-loading17; this is typical of incomplete distal RTA. Subsequently, various cases of type 1 RTA have been described in adults with non-autoimmune hypothyroidism.18,19 One of these patients presented with hyperkalaemia (type 4 RTA) compatible with an increase in Na+ levels in the tubular lumen of the collecting duct and impaired distal secretion due to an H+ and K+18 gradient defect.
However, most patients with both clinical conditions presented autoimmune hypothyroidism. This combination has been described both in adults20–22 and children23,24; in one of these cases of RTA, serum levels of T3, T4 and TSH were normal,22 supporting the notion that, as in other autoimmune diseases, antithyroid antibodies can per se can have a negative effect on renal acidification capacity.6,7 There are several references in the literature to the triple association of RTA, hypothyroidism and another autoimmune disease such as diabetes mellitus25 or Sjögren's syndrome.26,27 The fact that our second case was diagnosed with RTA 2 years after starting treatment with thyroid hormone supports the hypothesis that antithyroid antibodies have a negative effect on renal acidification capacity, although poor compliance with replacement treatment cannot be entirely ruled out. The pathophysiological mechanism of distal RTA associated with autoimmune disease is not well understood. In patients with Sjögren's syndrome, immunohistochemical studies have shown the absence of vacuolar H+-ATPase in collecting duct cells obtained from renal biopsies.28 More recently, in the same disease, autoantibodies against carbonic anhydrase II enzymes have been described (Fig. 2).29
The test used to determine maximum urinary pCO2 in both our patients is a variant of the one previously described by our Group. In this challenge, acetazolamide and sodium bicarbonate are administered simultaneously at lower doses than usual, thus obtaining valid results (urinary HCO3−>80mEq/l) while avoiding significant side effects.4,30,31
The loss of proximal bicarbonate reported in case 2 must be associated with a reduction in the activity of both the Na+/H+ exchanger (NHE3) and the Na+/HCO3− (NBCe1) cotransporter, as described above (Fig. 2).12 However, being a type 3 RTA (proximal and distal) the patient could, theoretically, be a carrier of autoantibodies against carbonic anhydrase II, which is functional in both the proximal and distal portions of the nephron.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Guerra-Hernández NE, Ordaz-López KV, Vargas-Poussou R, Escobar-Pérez L, García-Nieto VM. Acidosis tubular renal distal en dos niñas diagnosticadas de hipotiroidismo adquirido. Nefrologia. 2018;38:655–659.