Kidney transplant is the best therapy to manage end-stage kidney failure. The main barriers limiting this therapy are scarcity of cadaveric donors and the comorbidities of the patients with end-stage kidney failure, which prevent the transplant. Living kidney donor transplant makes it possible to obviate the problem of scarcity of cadaveric donor organs and also presents better results than the cadaveric transplant. The principal indication of living kidney donor transplant is preemptive transplant. This will allow the patient to avoid the complications of dialysis and it has also been demonstrated that it has better results than the transplant done after dialysis has been initiated. Priority indications of living donor transplant are also univitelline twins and HLA identical siblings. We will also have very favorable conditions when the donor is young and male. On the contrary, the living donor transplant will have worse results if the donors are over 60-65 years and the recipients are young, this possibly being a relative contraindication. There is an absolute contraindication for the living donation when the recipient has diseases with high risk of aggressive relapse in the grafts: focal and segmental hyalinosis that have had early relapse in the first transplant; atypical hemolytic uremic syndrome due to deficit or malfunction of the complement regulatory proteins; early development of glomerulonephritis due to anti-glomerular basement membrane antibody in patients with Alport Syndrome; primary hyperoxaluria.
El trasplante renal es la mejor terapia para hacer frente a la insuficiencia renal terminal. Las principales barreras que limitan esta terapéutica son la escasez de donantes fallecidos y las comorbilidades de los enfermos con insuficiencia renal terminal, que impiden el trasplante. El trasplante renal de vivo permite obviar el problema de la escasez de órganos de donante fallecido y además presenta mejores resultados que el trasplante de cadáver. La principal indicación del trasplante renal de vivo es el trasplante anticipado (preemptive). Éste permitirá al paciente librarse de las complicaciones de la diálisis y, además está demostrado que tiene mejores resultados que el trasplante realizado cuando ya se ha iniciado la diálisis. Son también indicaciones prioritarias de trasplante renal de vivo los gemelos univitelinos y los hermanos HLA idénticos. Además, tendremos condiciones muy favorables cuando el donante es joven y hombre. Por el contrario, el trasplante de vivo tendrá peores resultados si los donantes son mayores de 60-65 años y los receptores son jóvenes, pudiendo constituir esto una contraindicación relativa. Existe contraindicación absoluta para la donación de vivo cuando el receptor presenta enfermedades con alto riesgo de recidiva agresiva en los injertos: la hialinosis segmentaria y focal que han tenido una recidiva precoz en un primer trasplante; el síndrome hemolítico-urémico atípico por déficit o mala función de las proteínas reguladoras del complemento; el desarrollo precoz de una glomerulonefritis por anticuerpos antimembrana basal glomerular en pacientes con síndrome de Alpont, o la hiperoxaluria primaria.
INTRODUCTION
When patients are faced with end-stage renal failure, the best treatment option, without a doubt, is kidney transplantation before starting any form of dialysis. The scarcity of organs from cadaveric donors and the comorbidity of these patients, which contraindicates transplantation, prevent this treatment from being routinely performed prior to dialysis. Living-donor kidney transplantation can meet this objective perfectly, as it does not depend on waiting times imposed by cadaveric donation. In recent years, the expansion of genetically unrelated living donation has facilitated living-donor kidney transplantation as spouses, distant relatives, friends and even Good Samaritans have increased the pool of potential living donors. The results of this type of living-donor transplant have been better than those of cadaveric-donor transplants and the same as those for related living donors, despite worse HLA compatibility.1,2
Overall, living-donor kidney transplantation offers better survival than transplantation from cadaveric donors.1,3 The 2008 OPTN-UNOS (Organ Procurement and Transplantation Network-United Network for Organ Sharing) registry contains data from 159,119 transplants from cadaveric donors and 83 471 from living donors reported during the 20-year-period between 1988 and 2007. According to this data, the actuarial graft survival rates at 15 years were 25%-29% for cadaveric-donor transplantation and 42% for living-donor transplantation.1 The main reasons for these numbers are that living donors are thoroughly studied and selected from healthy individuals and they organs are not exposed to haemodynamic instability, sepsis, or nephrotoxic agents, as are those of cadaveric donors during brain death. Moreover, they do not suffer the deleterious effects of brain death and they have short cold ischaemia times before implantation.
These factors make living-donor transplantation the preferred option for treating end-stage kidney failure. However, not all patients have relatives or close friends who are willing to donate a kidney, and in many cases, although a donor may be available, the donor may not be optimal for ensuring long-term survival of the graft. Therefore, due to the morbidity to which the donor is exposed, we are obliged to ensure maximum success of the transplant in the short and long term when indicating this procedure.
Rather than discuss donor diseases that contraindicate living donation, which will be covered in a separate chapter on donor studies, this article deals with the situations that affect donor-recipient pairs in which the procedure is or is not recommended, according to the short and long-term results.
In general, provided that factors such as age and weight differences between donor and recipient are the same, living-donor kidney transplantation offers better short- and long-term graft survival rates than those from cadaveric donors. Therefore, if a patient has a living donor of a similar age, this option is preferable to cadaveric-donation.
However, if the living donor is elderly (e.g., older than 60 or 65 years) and the recipient is young (under 40 years), the results will be worse in terms of long-term graft survival and renal function, even if the donor still has perfect renal function with no cardiovascular risk. Although there is no absolute contraindication, there is a relative one and, in any case, the donor and recipient need to know this information.
Transplantation is the best option for a patient with onset of end-stage kidney failure, as long as the patient has no contraindications for transplantation (uncontrolled cancer, atherosclerosis with unresolved ischaemia in different locations, atherosclerosis that makes vascular anastomosis impossible and uncontrolled active infections). We will therefore describe in detail the circumstances in which living-donor kidney transplantation is better, similar and worse than cadaveric-donor transplantation, in terms of long-term survival (Table 1). Very few studies have addressed this issue from this perspective. In general, most studies on transplantation mix living- and cadaveric-donor transplantations, which makes it difficult to draw conclusions.
PRE-DIALYSIS TRANSPLANTATION
Kidney transplantation prior to dialysis, also known as pre-emptive kidney transplantation, is the optimal treatment strategy for dealing with end-stage kidney failure. Unfortunately, Spain has a high rate of cadaveric donors and living kidney donation is often associated with unnecessary morbidity for the healthy individual. The pre-dialysis nephrologists (except for the paediatric ones) are therefore not sufficiently aware of this treatment so as to convincingly present this option to patients with onset of end-stage kidney failure.
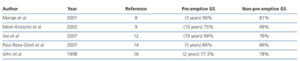
The benefits of pre-emptive kidney transplantation are clearly documented in kidney transplantation registries and the various studies of a particular centre (Table 2). The initial results on pre-emptive kidney transplants published in the nineties with both living and cadaveric donors showed better graft survival than those performed after starting dialysis.4 With pre-emptive transplantation, dialysis-associated morbidity is avoided, there is a low incidence of delayed graft function, the risk of acute rejection is lower, there is lower mortality and graft survival is improved. This was demonstrated by the analysis of 73 103 first transplants in adults from 1988 to 1997 in the United States Renal Data System Registry. The analysis showed that death with functioning a graft and death-censored graft survival were better in pre-emptive transplants and in patients who spent less time on dialysis.5
It has been speculated that the improved results of pre-emptive transplantation may be due to patients with better residual function. However, recent studies found no relationship between residual function at the time of pre-emptive transplantation and function at six months after transplantation,6 or in the annual decline in graft function when comparing pre-emptive and non-pre-emptive transplant patients.7 This suggests that the function achieved by the graft in pre-emptive transplantation is independent of its residual function, and that the improved survival of these transplants is independent of this function. These data support policies for indicating pre-emptive transplantation when dialysis is indicated, with no need to indicate it until compromised glomerular filtration begins to cause symptoms. From a practical standpoint, pre-dialysis nephrology visits should indicate pre-emptive living-donor transplantation when they believe, due to chronic and symptomatic renal function deterioration, that it is necessary to perform an arteriovenous fistula or a peritoneal catheter implantation in order to start haemodialysis or peritoneal dialysis (generally when the glomerular filtration rate is below 15ml/min). Obviously, transplantation will spare the need for performing these procedures. Nevertheless, from the earliest stages of kidney failure, patients should be made aware of the possibility for pre-emptive living-donor transplantation so that they can identify potential donors among relatives and friends.
Time on dialysis waiting for a transplant is associated with worse graft evolution, both for living and cadaveric donations. An analysis of living-donor graft survival in recipients older than 18 years used data from the U.S. Renal Data System from 1994 to 1997, and compared the evolution of 1819 pre-emptive living-donor transplants with 6662 living-donor transplants in patients who had already started dialysis. The analysis reported that graft survival at three years (uncensored for death) was 90% for pre-emptive transplant patients and 81% for those that had already started dialysis.8
We compared the evolution of 2405 paired kidneys (from the same donors) recorded in the U.S. Renal Data System database between 1988 and 1998. They were transplanted to patients with more than two years and less than six months on dialysis, and graft survival (non-adjusted and censored for patient deaths) at five and ten years was significantly worse in recipients of paired kidneys who were on dialysis for more than two years (58% and 29%, respectively) when compared to recipients who were on dialysis for less than six months (78% and 63%, respectively; P<.001 each one). For living-donor transplants, the adjusted survival rate at 10 years was 75% for pre-emptive transplants, compared to 49% for transplants in patients on dialysis for more than 24 months. In conclusion, time on dialysis is a modifiable risk factor for transplant evolution. Better survival outcome for living-donor transplantation may partly be due to it being performed sooner, and the patient waiting less time on dialysis. In fact, this study showed that survival for cadaveric-donor transplant patients who waited less than six months on dialysis was equivalent to living-donor transplant patients who have been on dialysis for more than two years.9
The American registry data showed that 25% of living-donor kidney transplants between 1994 and 2002 were pre-emptive transplants. Moreover, among recipients of cadaveric-donor kidney transplants, 7% received them pre-emptively. Pre-emptive transplants had better graft and patient survival. Pre-emptive transplant recipients had a lower incidence of post-transplant dialysis and acute rejection episodes prior to hospital discharge. Therefore, those patients with an appropriate living donor, should be transplanted before starting dialysis or, if dialysis has already begun, as soon as possible.10
According to the Australian registry, data on 2739 first kidney transplants (between 1980 and 2004) in recipients under 30 years old showed that for adolescents, time on dialysis affects graft survival at five and ten years. In contrast, pre-emptive transplantation achieved the best results, as it reduced the risk of graft loss by 50% in the long term.11
As for the results of a single centre for living-donor kidney transplantation, some cases have been reported showing better or equal results for pre-emptive transplants compared to those performed in patients already on dialysis. Among those that report better results for pre-emptive transplantation, of note is a Korean series that compared 63 pre-emptive living-donor kidney transplants with 359 living-donor transplants performed after starting dialysis. They found that graft survival rates at 10 years were significantly better in the pre-emptive group than in the dialysis group (94% compared to 76%), although patient survival rates were no different in for time period (98% compared to 91%).12 A French study of 44 pre-emptive transplants (16% living donor) also found a 93% graft survival rate compared to 77% for a group of 419 (2% living donor) performed on patients on dialysis. This was recorded at the end of a follow-up that varied between 46 months for the pre-emptive group and 63 months for the post-dialysis group.13 Among those that found no differences was a single Iranian centre that compared 300 pre-emptive living-donor kidney transplants with 300 living-donor transplants in patients on dialysis. Survival at five years both for graft (84% for pre-emptive compared to 89% for post-dialysis) and patient (93% compared to 97%) were comparable.14 An Egyptian centre reported that of 1279 living-donor transplants performed between March 1976 and March 2001, 82 (6.4%) were pre-emptive. Results at five years for these last two centres were similar to the 1197 transplants in patients who had already started dialysis, but pre-emptive transplantation eliminated the complications, inconvenience and cost of dialysis.15 A single centre Indian study compared 43 pre-emptive living-donor kidney transplants with 83 living-donor control transplants that were performed after starting dialysis, between 1989 and 1996. Survival rates at one and two years were similar for both the graft (pre-emptive: 82.8% and 77.3%; controls: 82% and 78%, respectively) and patient (pre-emptive: 92% and 89.5%; controls: 91% and 89.5%, respectively). As such, pre-emptive transplantation eliminated the inconvenience of dialysis and was much less costly.16
In summary, most of the studies described above show the superiority of pre-emptive living-donor kidney transplantation over other transplantation methods. Even though some single centres that analysed fewer patients did not show differences between pre-emptive transplants and those performed after dialysis had started, in every case, pre-emptive transplantation succeeded in avoiding dialysis and all the inconvenience it entails.
LIVING-DONOR KIDNEY TRANSPLANTATION IN PATIENTS ON DIALYSIS
In many cases, the willingness of a living donor (relative or not) arises when the candidate for transplant on dialysis remains on the waiting lists indefinitely, with little chance of receiving a cadaveric-donor kidney transplant. The results of living-donor transplantation have improved decade by decade since the sixties, despite the expansion of living-donor acceptance criteria (mainly donation by the elderly or genetically unrelated individuals). The reasons for this improvement are the development of new immunosuppressants that control acute rejection better, new antibiotic and antiviral drugs that treat infections in these patients and the improved treatment of cardiovascular problems with the early detection and treatment of coronary artery disease and cerebrovascular events.
When approaching living-donor kidney transplantation in a patient on dialysis, one should understand the factors that determine worse evolution in order to avoid them as much as possible. The factors that worsened long-term survival in 2540 living-donor transplants performed at the University of Minnesota from 1963 to 1998 included: delayed graft function in living-donor transplants, acute rejection, the combination of these two conditions, pre-transplant cardiovascular disease, smoking, dialysis and a donor age over 55 years.17
Advantages and disadvantages of living-donor transplantation according to donor and recipient characteristics:
Young candidate on waiting list
Cadaveric donor quality has declined in recent years due to the effectiveness of road and workplace safety policies, which have drastically reduced the deaths of young people in traffic and job accidents. There is evidence that one of the main factors affecting long-term graft survival is donor age. It is crucial for young patients (under 60 years old) to find donors of similar age to ensure long-term graft survival. Meeting this criterion is difficult and waiting times for receiving this type of cadaveric-donor graft are very long.
Living donor of a similar age as the recipient
This condition (young living donor for young recipient) ensures better survival than if the recipient receives a kidney from an elderly cadaveric donor. In addition, the patient will have the general advantages of a living-donor transplant such as shorter cold ischaemia time and immediate renal function. All of these conditions, as well as HLA compatibility if patient and donor are related, will create the ideal situation for a long survival.
Elderly living donor
Sometimes living donors are elderly, as in the case of parents. Kidney transplants from elderly cadaveric donors have worse survival rates because the grafts have a lower nephron mass, are senescent and are more susceptible to ischaemic attacks and acute rejection events. What has been described here for cadaveric donors may also apply to living donors, although it has not been widely studied in medical literature.
In these circumstances (elderly living donors and young recipients), transplantation is not contraindicated but the donor and the recipient must understand that survival rates are lower. In contrast with elderly cadaveric-donors and young recipient, this combination entails some advantages, such as shorter cold ischaemia times and better HLA compatibility. There are no studies comparing graft survival in young people with young cadaveric donors and elderly living donors, but data from various registries and centres seem to show that survival would be greater with young cadaveric donors. Nevertheless, living-donor transplantation would avoid the delay in graft function and therefore improve the evolution of this type of pairing. It is unclear what to advise a patient when taking into account that the time on dialysis negatively influences the survival of patients and future grafts.5,9,18 Therefore, clinicians must balance all these factors when making decisions and informing patients of them.
An American registry, in an analysis of 73 073 first kidney transplants performed between 1995 and 2003, showed that elderly (>55 years) living-donor transplants were conducted pre-emptively on elderly white female recipients. In addition, they were performed more often between spouses than between relatives and even more so when the husband was the donor. Glomerular filtration at one year was inversely proportional to the age of the living donor at the time of donation. The multivariate analysis on graft loss risk with living donors between 55 and 64 years old was similar to that of cadaveric donors under 55 years, and it was higher when the living donor was between 65 and 69 years (HR=1.3; 95% CI: 1.1-1.7) or were over 70 years (HR=1.7; 95% CI: 1.1-2.6). The conclusion is that donors younger than 65 years may be living donors with advantages over younger cadaveric donors despite achieving worse glomerular filtration rates at one year than younger living-donor transplants.19
These data are consistent with the UK Transplant Registry, which analysed the factors affecting long-term graft and patient survival. They studied data from 3142 living-donor transplants (71% genetically related and 29% unrelated) performed between 2000 and 2007 inclusive. They found that HLA (-A, -B, and -DR) incompatibility did not have a negative effect, but those patients who received a graft from donors who were over 59 years had lower survival rates. Furthermore, being a female recipient was also an independent risk factor for worse survival.20
A multivariate analysis of a Norwegian registry with 739 living-donor kidney transplants performed between 1994 and 2004 also found that donor age over 65 years was a risk factor for graft loss for all time periods after transplantation.21 In these latter studies, the age of the donor was not adapted to the recipient's.
An observational study analysed a cohort of kidney transplant recipients aged 60 years or older who underwent transplantation between 1996 and 2005 and were included in The Organ Procurement Transplant Network/United Network for Organ Sharing American registry. The study focused on the results for living donors over 55 years old. In these elderly recipients of kidneys from living donors over 55 years old, although there was lower graft survival at three years compared to those who received kidneys from younger living donors, patient survival was similar. Furthermore, graft and patient survival rates were greater than in recipients who received kidneys from cadaveric donors of any age. This was especially noticeable when compared to kidney transplants from expanded criteria donors.22 Therefore, being an elderly kidney transplant candidate is the ideal situation to have an elderly living donor.
Female living donor or low-weight donor
In kidney transplantation, donor age and graft size are known factors that influence the long-term evolution of the graft.23-26 Women tend to have smaller kidneys with 17% less nephrons than men. The number of nephrons per kidney is positively correlated with the weight of the kidney and negatively correlated with the age of the individual.24 It has been reported that kidneys from female donors that are transplanted to men have worse evolution.20,27-29
Kwon et al30 assessed the impact of age and sex on the results of living-donor kidney transplantation. Their series of 614 living-donor kidney transplants were divided into four groups according to the four combinations of sex between donor and recipient. The group with the worst survival was female donors whose kidneys were transplanted to male recipients. Graft survival at five years was 75% compared to 83%-85% for the other three groups. A risk factor analysis performed as part of the study found that factors that influence worse long-term graft evolution were donor age, female sex, acute rejection and HLA incompatibilities.
Lankarani et al came to similar conclusions when analysing a series of 2649 first unrelated living-donor transplants. They observed worse survival rates for transplants from female donors to male recipients, and among young people who received kidneys from older donors. They found that using kidneys from young donors (under 40 years) and avoiding female donors for males is the optimal condition for living-donor transplantation.31
Other analyses suggest that the negative effect female donors have on male recipients not only has to do with the difference in the number of transplanted nephrons but is also related to the fact that the female graft would trigger a greater immunological response.21,32 Female sex has even been suggested to be a risk factor for early acute rejection.21
Overall, current evidence tells us that when the donor is an elderly female and the recipient is a young male, we do not have the most appropriate circumstances for ensuring good medium and long-term results. Therefore, this donor-recipient pairing would be a relative contraindication for living-donor kidney donation. As such, if living-donor transplantation is decided upon using this type of donor, it should only be performed after comprehensively informing the donor and the recipient about the risks.
In general, these unfavourable conditions would be less important for pre-emptive transplantation. The advantages of not implementing dialysis probably outweigh, at least in part, the disadvantages of these types of donor-recipient pairs.
DISEASES WITH HIGH RATES OF RECURRENCE IN KIDNEY TRANSPLANTATION
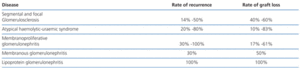
Patients with kidney diseases with high rates of recurrence after transplantation are absolutely contraindicated for living-donor kidney donation. The diseases may be relative contraindications in the first transplant but if there is a recurrence of primary kidney disease, and this is the cause of graft loss, the contraindication is absolute for the second transplant. The processes that are absolutely contraindicated are segmental or focal hyalinosis with early recurrence in the first transplant, atypical haemolytic-uraemic syndrome due to deficit or dysfunction of complement regulatory proteins,33,34 early development of glomerulonephritis due to anti-glomerular basement membrane antibodies in patients with Alport’s syndrome and primary hyperoxaluria. In these circumstances, living-donor kidney transplantation is contraindicated although cadaveric-donor transplantation or double transplantation of liver and kidney may be a good treatment option.
There are many other diseases that recur in the transplant35 and if the rates of graft loss due to these recurrences are high, the clinician must consider setting a relative contraindication for living-donor transplantation. Table 3 summarises the diseases susceptible to recurrence.
MONOZYGOTIC (IDENTICAL) TWINS
This is without doubt the ideal situation for living-donor transplantation since it almost guarantees a definitive solution to the recipient's kidney problem with little or no immunosuppression. The first kidney transplantation between humans was performed successfully between monozygotic twins and although this was before immunosuppressants were available, the genetic similarities guaranteed long-term graft survival.36,37
The results of these transplants between identical twins have been recently evaluated in the U.S.A and Britain.38 Transplant data came from U.S.A and British registries for the 1988-2004 period. In the U.S.A, 120 cases were found while Britain had 12. Graft survival was excellent at one, three and five years (99.17%, 91.84% and 88.96%, respectively in the U.S.A, and 83.3%, 83.3% and 75%, respectively in the British group). It was noteworthy that a large number of patients maintained some form of immunosuppression, usually because of doubts about whether the twins were monozygotic. Thus, genetic studies to determine whether twins are monozygotic help eliminate immunosuppression.39
HLA-IDENTICAL SIBLINGS
A HLA-identical sibling is another favourable situation for living-donor transplantation, although it is not as immunologically neutral as monozygotic twins. An analysis by De Mattos et al of 108 living-donor transplants between HLA-identical siblings performed at their institution between 1977 and 1993, observed an acute rejection incidence of 46%, although it should be noted that modern immunosuppression was not used. Patients who had acute rejection had worse long-term evolution (69% at five years compared to 88% in the overall series), as well as those patients who suffered kidney failure due to diseases that could potentially recur in the transplant.40 To summarise, monozygotic twins, and to a lesser extent HLA-identical siblings, are an ideal situation for living-donor kidney transplantation and under these circumstances transplantation is especially indicated.
HYPERIMMUNISED PATIENTS
These patients may benefit from living donations from HLA-identical siblings, those that share a haplotype or parents. If there are positive crossmatches with all relatives then the ideal situation would be to enter into a hyper-immunised patient kidney transplant programme sharing cadaveric donors or crossover living-donor kidney transplant programmes. Prior to this, patients can be administered desensitisation treatments to see whether the crossmatch with living donors comes back negative.
PANCREAS AND KIDNEY TRANSPLANTATION CANDIDATES
The best treatment for patients with type 1 diabetes mellitus and end-stage kidney failure is simultaneous transplantation of pancreas and kidney, and the ideal situation is pre-emptive transplantation with organs from the same cadaveric donor. Unfortunately, the shortage of pancreas donors is very pronounced, given that the selection criteria specify very young donors with hardly any acute comorbidity. This means that patients spend long periods on dialysis waiting for a simultaneous transplant.
An alternative to simultaneous transplantation of pancreas and kidney for type 1 diabetics with kidney failure is sequential transplantation of a kidney from a living donor followed by a pancreatic transplant from a cadaveric donor. This treatment strategy makes pre-emptive living-donor transplantation possible and avoids the morbidity of dialysis. Poommipanit et al in their analysis of the Organ Procurement Transplant Network/United Network of Organ Sharing Database reported results from this strategy, comparing 807 pancreatic transplants performed after a living-donor kidney transplant with 5580 transplants performed simultaneously with organs from cadaveric donors. Patient and kidney survival were greater in transplants performed after a living-donor kidney transplant, although hospital stays and pancreatic transplant survival were favourable to simultaneous transplantation.41
This greater patient and kidney graft survival was confirmed by other studies in which patients who received a pancreas after the living-donor kidney transplant had better patient and kidney graft survival than those who never received a pancreatic transplant.42 In some studies, even living-donor kidney transplantation in diabetic patients achieved better kidney graft survival than simultaneous transplantation of pancreas and kidney. This was due to the time saved from dialysis in these diabetic patients with high cardiovascular risk.43
To summarise, living-donor transplantation in type 1 diabetic patients should be seen as a priority, without conflicting with the latter indication for pancreatic transplantation after the living-donor kidney transplant. If it is put into practice, kidney transplantation should be located primarily in the left iliac fossa to facilitate later surgery for pancreatic transplantation in the right iliac fossa.
Table 1. Absolute and relative indications and contraindications for living-donor kidney transplantation
Table 2. Results of pre-emptive living-donor kidney transplants
Table 3. Recurrent diseases in kidney transplantation(ref. 35)