Initiation of peritoneal dialysis (PD) with 3 exchanges has become common practice in recent years, despite the lack of published clinical data.
ObjectiveTo describe experience with incremental peritoneal dialysis (IPD) at a single site.
Material and methodsA total of 46 IPD patients undergoing 2-year clinical, laboratory, treatment and progression follow-up.
ResultsTo 25% of patients were transplanted on IPD. Mean time on IPD before transfer to conventional PD of 24 months, half of the patients because of fluid balance. Good clinical and biochemical results with a peritonitis rate of one episode per 99 months. There was an improvement in the loss of residual kidney function compared to the pre-dialysis period (−7.06 vs. −1.58ml/min/year; P=.0001).
ConclusionsIPD with 3 peritoneal exchanges offers good results. Most patients remain stable during the first 2 years and there is an improvement in the loss of residual kidney function compared to the pre-dialysis period.
En los últimos años el inicio de diálisis peritoneal (DP) con 3 recambios se ha convertido en una práctica habitual, aunque se dispone de pocos resultados clínicos publicados.
ObjetivoDescripción de la experiencia de inicio con DP incremental (DPI) en un centro.
Material y métodosA 46 pacientes en DPI se les realizó seguimiento clínico, analítico y tratamiento, y se estudió su evolución a 2 años.
ResultadosA un 25% de los pacientes se les trasplanta en DPI. Tiempo medio de transferencia a DP convencional de 24 meses. La mitad de los pacientes son transferidos por manejo de líquidos. Buena estabilidad clínica y analítica con tasa de peritonitis de un episodio cada 99 meses. Enlentecimiento de la pérdida de función renal residual respecto al período prediálisis (−7,06 vs. −1,58ml/min/año; p=0,0001).
ConclusionesLa experiencia en DPI con 3 recambios de inicio es positiva. La mayoría de los pacientes se mantienen estables durante los 2 primeros años, con un enlentecimiento de la pérdida de función renal residual respecto el período prediálisis.
In recent years it has been proposed to start peritoneal dialysis (PD) using the modality of incremental peritoneal dialysis (IPD). This is defined as starting PD with 3 or fewer peritoneal exchanges per day. The IPD modality is postulated as an option for patients who start dialysis before manifestations of severe uraemia are present.1,2
By performing fewer exchanges per day, patients on IPD would have a reduced risk of peritonitis and a better quality of life. Furthermore, by reducing peritoneal glucose exposure and absorption, the peritoneal membrane should be better preserved, with less chronic inflammation and alterations in carbohydrate metabolism. All this would contribute to preserve future vascular accesses,3–5 in case there is a need to switch to haemodialysis (HD). It has also been reported that acceptable levels of solute clearance are maintained in IPD and there is an adequate control of other biochemical parameters, while residual renal function (RRF) is preserved. It should be highlighted that one study shows that in IPD there is slowing-down in the rate of renal function loss as compared to that observed during the pre-dialysis period.6
Another study compares the evolution of patients who received transplantation during the first 3 years on dialysis. As compared with HD patients, IPD patients have a lower incidence of delayed graft function and better long-term clinical results, both for the patient and the graft.7
Lastly, the possibility of starting treatment with IPD encourages patients to choose the modality of PD more often, particularly continuous ambulatory peritoneal dialysis (CAPD).6
The increased number of patients starting a pre-scheduled renal replacement therapy (RRT) and the potential advantages of IPD have led many PD centres to choose this modality in recent years. Nevertheless, there is limited evidence on the benefits and on the clinical management of these patients. Few studies analysing IPD are available and most of them are retrospective with short number of patients, no control group and carried out in the same country, which means the results cannot be extrapolated to other populations on dialysis.
Therefore, the objective of this study is to analyse the clinical and analytical characteristics of patients during the 2 first years on IPD, as well as patients prognosis and outcomes.
Material and methodsDesign: descriptive, longitudinal and retrospective study.
PatientsWe included all patients from the Lleida Hospital Arnau de Vilanova PD unit who started CAPD using IPD, that is, 3 or fewer exchanges per day. We excluded patients who started CAPD using conventional treatment (4 exchanges) or those on automated peritoneal dialysis (APD).
DataThe study period started in August 2003 (date when the first IPD patient was included in the study) and ended in December 2012 (final data collection date).
The data collected included variables on demographics, anthropometric and analytical data and dialysis and pharmacological treatment data (number of antihypertensives, number of phosphate chelating agents, use of ACE inhibitors or ARBs, diuretics, paricalcitol and cinacalcet and darbepoetin doses). Comorbidity was estimated using the Charlson index evaluated for PD unadjusted by age.8 Adequacy was calculated using Kt/V (renal, peritoneal and total) and RRF using combined 24h urinary urea and creatinine clearances. These data were collected at the start of IPD, and at 6, 12, 18 and 24 months of IPD treatment. In the case of RRF, progression was also recorded in the last predialysis year for stable patients with a minimum of 4 valid determinations. Peritoneal function was estimated using the peritoneal equilibration test at the start of the modality and it was repeated each year. The rate of peritonitis was also calculated for the first 2 years on IPD.
Regarding the evolution on this modality, we recorded time on PD, on IPD and reasons for suspension of IPD, renal transplant, deaths, switching to HD, clinical manifestations of uraemia, Kt/V<2 without clinical manifestations of uraemia, fluid overload and switching to APD due to patient's choice.
StatisticsThe Kolmogorov–Smirnov test was used to determine normal distribution of continuous variables. We expressed quantitative variables as means with standard deviation. Bivariate comparisons were performed using the Student's t-test for paired samples. Comparisons between qualitative variables, expressed as frequency or percentage, were performed with the test χ2. Results were considered to be significant with a p-value <0.05 (95% confidence interval).
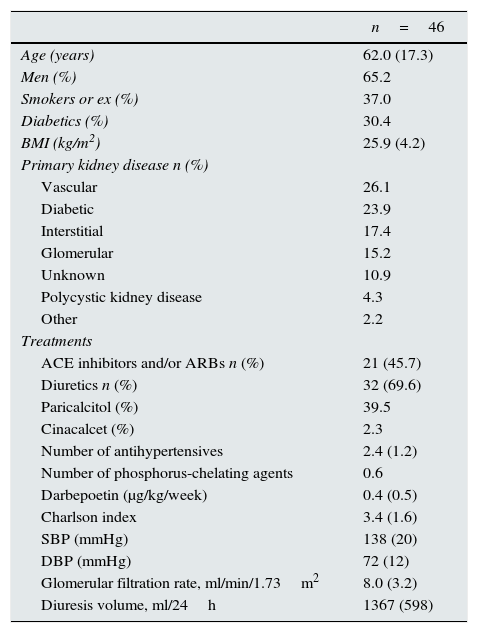
ResultsDuring the study period, 75 patients were included in the PD programme; of these, 28 patients were excluded for choosing APD and one patient for starting the conventional modality due to a medical history of severe heart disease. A total of 46 patients started the IPD, of whom 39 (84.8%) came from the multidisciplinary advanced kidney disease clinics (MAKDC), 4 (8.7%) came from hemodialysis HD and 3 (6.5%) had renal transplant with poor renal function. Table 1 shows the baseline characteristics of the patients (epidemiological, anthropometric, RRF and pharmacological treatment).
Clinical data and baseline treatment.
| n=46 | |
|---|---|
| Age (years) | 62.0 (17.3) |
| Men (%) | 65.2 |
| Smokers or ex (%) | 37.0 |
| Diabetics (%) | 30.4 |
| BMI (kg/m2) | 25.9 (4.2) |
| Primary kidney disease n (%) | |
| Vascular | 26.1 |
| Diabetic | 23.9 |
| Interstitial | 17.4 |
| Glomerular | 15.2 |
| Unknown | 10.9 |
| Polycystic kidney disease | 4.3 |
| Other | 2.2 |
| Treatments | |
| ACE inhibitors and/or ARBs n (%) | 21 (45.7) |
| Diuretics n (%) | 32 (69.6) |
| Paricalcitol (%) | 39.5 |
| Cinacalcet (%) | 2.3 |
| Number of antihypertensives | 2.4 (1.2) |
| Number of phosphorus-chelating agents | 0.6 |
| Darbepoetin (μg/kg/week) | 0.4 (0.5) |
| Charlson index | 3.4 (1.6) |
| SBP (mmHg) | 138 (20) |
| DBP (mmHg) | 72 (12) |
| Glomerular filtration rate, ml/min/1.73m2 | 8.0 (3.2) |
| Diuresis volume, ml/24h | 1367 (598) |
Values expressed as percentages or mean and (standard deviation).
SBP, systolic blood pressure; DBP, diastolic blood pressure; ACE inhibitors, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers.
All patients started IPD treatment with 3 exchanges per day, at a volume ranging from 1500 to 2000ml per exchange. The treatment always included an icodextrin exchange (Extraneal®) and in 18 (39.1%) of the patients an amino acid exchange (Nutrineal®). The other exchanges were with the dialysis solution Physioneal® at a glucose concentration of 1.36%.
The mean time on IPD was 22.5±14 months (range: 6–61). During follow-up, 11 (24%) patients received transplantation while they were on IPD, 3 patients were switched to HD (2 due to severe peritonitis and one due to an unresolved alteration in the abdominal wall), 2 patients died (due to terminal multiple myeloma and disseminated cancer with pneumonia) and 22 (47.8%) of the patients were switched to conventional PD. The mean time on IPD in the subgroup of patients switched to conventional PD was 24.5 months. The reasons for the switch to conventional PD were: fluid overload in 9 (40.9%) patients; Kt/V<2 without clinical manifestations of uraemia in 7 (31.8%) patients; clinical manifestations of uraemia in 5 (22.7%) patients; and preference of APD in one patient. At the end of the study period, 8 (36.36%) patients continued on IPD.
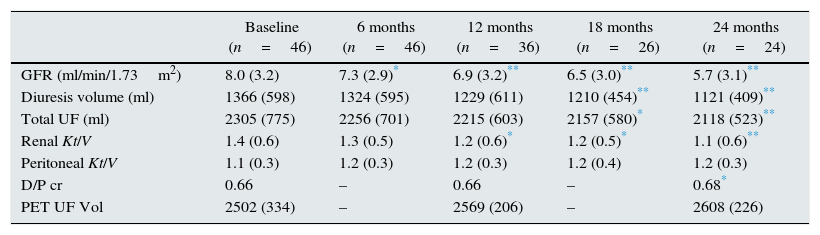
Table 2 shows the progression of RRF, adequacy and peritoneal function parameters in the first 2 years of IPD follow-up. During this period there was a decrease in glomerular filtration rate (GFR) of around 1.5ml/min/year, accompanied by a decrease in renal Kt/V. Total fluid loss also decreased due to a reduction of diuresis. Comparison of the annual loss of renal function during pre-dialysis period with the first 2 years of IPD showed that the rate of renal function deterioration slowed down when patients started PD: −7.06ml/min/year before initiation of IPD vs. −1.58ml/min/year in IPD; p=0.0001. None of the patients who started IPD experienced anuria during the follow-up period. Regarding peritoneal function, an increase in solute transport was observed (D/P cr), which was significant in the second year, with no loss of UF capacity.
RRF progression, adequacy and PET.
| Baseline (n=46) | 6 months (n=46) | 12 months (n=36) | 18 months (n=26) | 24 months (n=24) | |
|---|---|---|---|---|---|
| GFR (ml/min/1.73m2) | 8.0 (3.2) | 7.3 (2.9)* | 6.9 (3.2)** | 6.5 (3.0)** | 5.7 (3.1)** |
| Diuresis volume (ml) | 1366 (598) | 1324 (595) | 1229 (611) | 1210 (454)** | 1121 (409)** |
| Total UF (ml) | 2305 (775) | 2256 (701) | 2215 (603) | 2157 (580)* | 2118 (523)** |
| Renal Kt/V | 1.4 (0.6) | 1.3 (0.5) | 1.2 (0.6)* | 1.2 (0.5)* | 1.1 (0.6)** |
| Peritoneal Kt/V | 1.1 (0.3) | 1.2 (0.3) | 1.2 (0.3) | 1.2 (0.4) | 1.2 (0.3) |
| D/P cr | 0.66 | – | 0.66 | – | 0.68* |
| PET UF Vol | 2502 (334) | – | 2569 (206) | – | 2608 (226) |
Compared to baseline value:
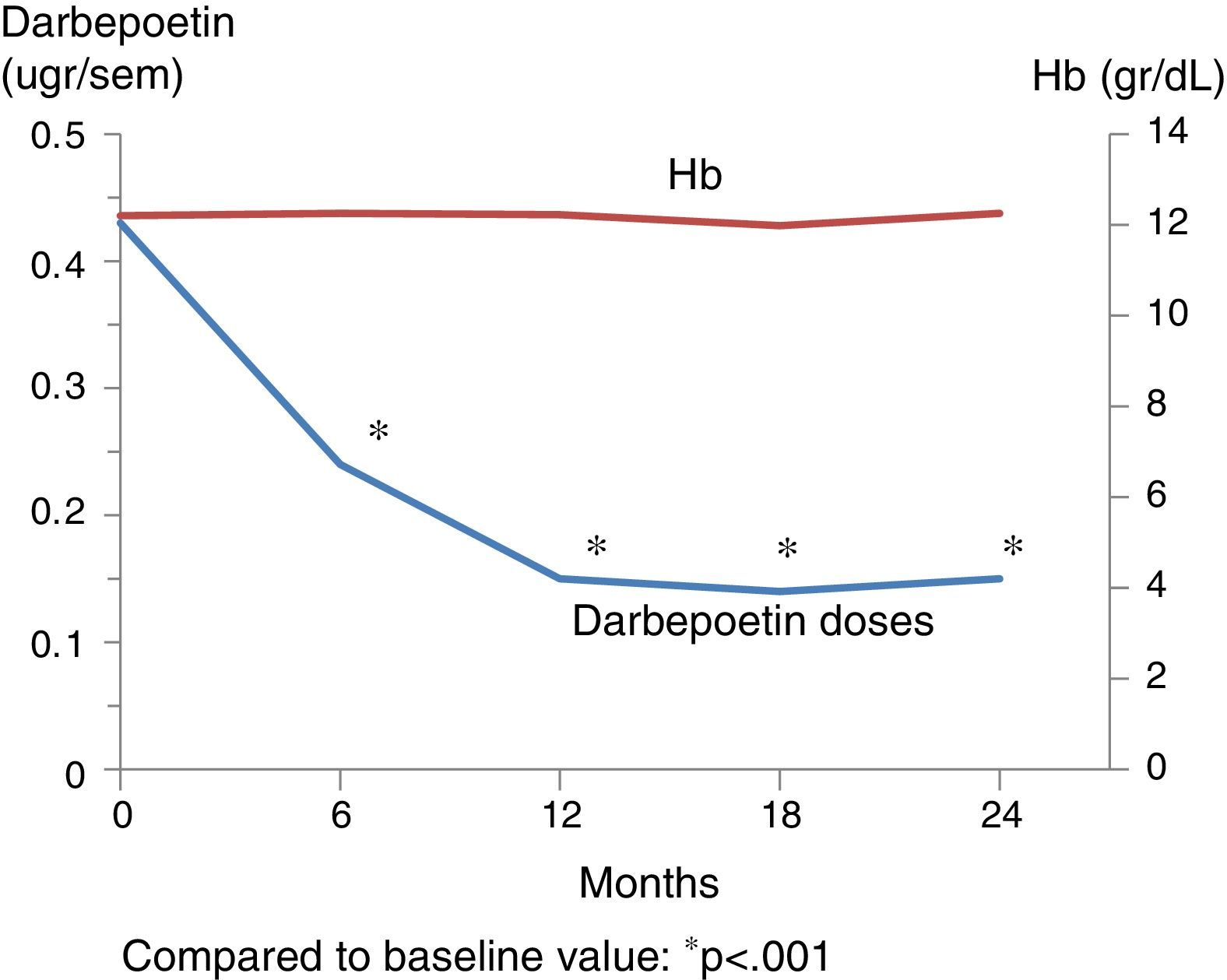
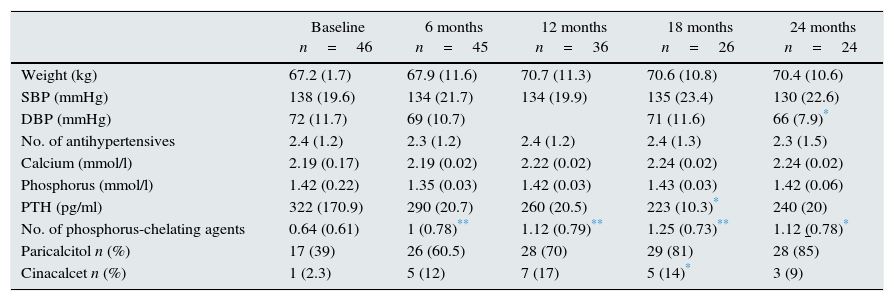
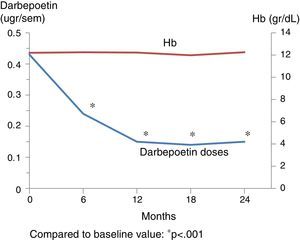
Table 3 shows the outcome of clinical, analytical and pharmacological parameters. The mean weight increased non-significantly; blood pressure (BP) did not change except for the diastolic BP that was decreased at 2 years. There were no significant changes in the number of antihypertensive drugs prescribed. Regarding mineral metabolism parameters, the serum calcium and phosphorus levels were stable with a tendency for intact parathormone levels to decrease. This was controlled through a significant increase in phosphorus chelating agents from baseline and an increase in the number of patients on treatment with paricalcitol and cinacalcet. Fig. 1 shows the progression of haemoglobin levels and darbepoetin dose. Haemoglobin levels remained stable, within acceptable ranges. It should be highlighted that these levels were maintained in spite of a significant reduction of darbepoetin doses prescribed throughout the follow-up period.
Outcome of clinical, analytical and pharmacological parameters.
| Baseline n=46 | 6 months n=45 | 12 months n=36 | 18 months n=26 | 24 months n=24 | |
|---|---|---|---|---|---|
| Weight (kg) | 67.2 (1.7) | 67.9 (11.6) | 70.7 (11.3) | 70.6 (10.8) | 70.4 (10.6) |
| SBP (mmHg) | 138 (19.6) | 134 (21.7) | 134 (19.9) | 135 (23.4) | 130 (22.6) |
| DBP (mmHg) | 72 (11.7) | 69 (10.7) | 71 (11.6) | 66 (7.9)* | |
| No. of antihypertensives | 2.4 (1.2) | 2.3 (1.2) | 2.4 (1.2) | 2.4 (1.3) | 2.3 (1.5) |
| Calcium (mmol/l) | 2.19 (0.17) | 2.19 (0.02) | 2.22 (0.02) | 2.24 (0.02) | 2.24 (0.02) |
| Phosphorus (mmol/l) | 1.42 (0.22) | 1.35 (0.03) | 1.42 (0.03) | 1.43 (0.03) | 1.42 (0.06) |
| PTH (pg/ml) | 322 (170.9) | 290 (20.7) | 260 (20.5) | 223 (10.3)* | 240 (20) |
| No. of phosphorus-chelating agents | 0.64 (0.61) | 1 (0.78)** | 1.12 (0.79)** | 1.25 (0.73)** | 1.12 (0.78)* |
| Paricalcitol n (%) | 17 (39) | 26 (60.5) | 28 (70) | 29 (81) | 28 (85) |
| Cinacalcet n (%) | 1 (2.3) | 5 (12) | 7 (17) | 5 (14)* | 3 (9) |
Compared to baseline value:
Lastly, of the population studied, 10 (21.7%) patients presented an episode of peritonitis during the first 2 years on IPD. The rate of peritonitis was one episode every 99 months, which is comparable to the rate presented by the rest of the population on PD in our unit during the same period.
DiscussionThis study shows that most patients starting IPD present considerable clinical and analytical stability during the first 2 years on this modality. Moreover, the loss of RRF slows down compared to the year prior to starting PD.
The definition of IPD in CAPD has not been clearly established. In most studies, IPD is defined as patient on PD with 2 or fewer manual exchanges1,9. However, in other studies,6,10,11 the incremental modality includes patients with 3 or fewer exchanges prescribed according to glomerular filtration rate or Kt/V. We chose a definition for IPD as starting PD with 3 or fewer exchanges because the classic and standard description of CAPD is 4 peritoneal exchanges,12 and this framework has been used for several decades. In recent years, many PD units have adopted 3 or fewer peritoneal exchanges when the clinical condition allows as the standard initial modality. Two facts have contributed to this change: the decrease in adequacy objectives13,14 and a greater number of patients with planned dialysis start thanks to MAKDC. Although it is a routine clinical practice, there is limited published data.
Our experience was largely positive. Firstly, because it shows that many patients starting PD can do it on IPD. Only in one case starting with only 3 exchanges was contraindicated due to, severe heart failure with several episodes of overhydration. Patients not included on IPD were those that chose APD as their starting modality. Nevertheless, there was a bias in the choice for APD because it was the modality of choice in a considerable number of patients who required a caregiver or who had been referred from HD or renal transplant. We do not have data on starting APD using an incremental modality, defined in general as fewer than 6 days of weekly treatment.
Secondly, our experience was positive because during their time on IPD, the patients remained clinically and analytically stable, with low peritonitis rates and did not require increases in prescribed medication. In fact, haemoglobin remained stable while darbepoetin doses had significantly been reduced. Values of BP remained within normal ranges without the need to increase antihypertensive treatment; thus, the non-significant increase in weight should not be attributed to overhydration. Lastly, serum calcium and phosphate concentration were maintained throughout follow-up, with a decrease in parathormone levels. In this case there was an increase in the number of patients on phosphate-chelating agents and treated with paricalcitol and cinacalcet. Notwithstanding, no patients had to be switched to standard CAPD or HD due to poor control of mineral metabolism.
A third aspect to be highlighted is the progression of RRF. Mean glomerular filtration rate (GFR) at the before initiation of IPD was 8ml/min. It should be noted that during the length of the study the recommendation of relatively RRT start was followed, as indicated by guidelines. Currently, after the results from the IDEAL study15 and other publications, the indication was to reconsider an early initiation of replacement therapy. Nonetheless, it should be remembered that in the IDEAL study, the mean GFR for the late start group was 9.2ml/ml, although it was calculated by estimating the creatinine clearance formula corrected for body surface. In any case, our patients did not start RRT with very high GFRs and, in any case, the loss of RRF slowed down from −7.06 to −1.58ml/min/year. This is surely key to explaining IPD patient stability during the 24 months that they remained on this modality. Viglino et al.6 also observed a reduction in the rate of renal function loss of 2.4ml/min/year with IPD compared to the predialysis period. This reduction in the loss of RRF cannot be attributed to the incremental modality, given that Berlanga et al.16 already reported this same effect with PD in general. However, it is important to demonstrate that the incremental modality also has this effect, given that the importance of preserving RRF in PD is beyond the clinical management; it is well known that this factor is also associated to lower morbidity and mortality in PD patients.17
Lastly, it is worth noting that one quarter of patients received transplant while they were still on the incremental modality. Patients who switched to the conventional modality were maintained on IPD for an average of 2 years in good conditions, and fluid management was the main reason for switching to conventional PD. However, overhydration was not severe and there were no cases requiring hospitalization. Thirty-two per cent (32%) of patients were switched to conventional PD due to a Kt/V<2, although they were entirely asymptomatic. This consideration should probably be reconsidered in light of the most recent results and publications; some of these patients could have been maintained on IPD without complications. In any case, individualised, case-by-case assessment and acquired experience will be crucial to optimise IPD and suitably switch to conventional PD.
In summary, we had a positive experience at our centre by starting PD with 3 peritoneal exchanges. Most patients remained stable for the first 2 years and one quarter of them received transplant while on this modality. Moreover, we confirmed a slow-down of RRF compared to the predialysis period.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Borràs Sans M, Chacón Camacho A, Cerdá Vilaplana C, Usón Nuño A, Fernández E. Diálisis peritoneal incremental: resultados clínicos y preservación de la función renal residual. Actas Urol Esp. 2016;36:299–303.