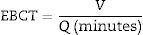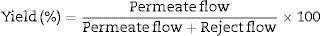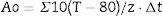A Best Practice Guideline about Dialysis fluid purity was developed under the leadership of the Spanish Society of Nephrology in 2004. The second edition revised Guideline considered new evidences and International Standard. The guideline has established recommendations for standards for preparing dialysate: water, concentrates and haemodialysis proportioning systems. This guideline is based on the ISO 13959, European Pharmacopoeia, the Real Farmacopea Española, the AAMI Standards and Recommended Practices, European Best Practice Guidelines for Haemodialysis, literature reviews, according to their level of evidence, and the opinion of the expert Spanish group.
Two levels of quality of water were defined: purified water and high purified water (ultra pure) and for dialysate: ultra pure dialysate. Regular use of ultra pure dialysate is recommended for all type of haemodialysis to prevent and delay the occurrence of complications: inflammation, malnutrition, anaemia and amiloidosis.
Water, concentrates and dialysate quality requirements are defined as maximum allowable contaminant levels: chemicals (4.1.2), conductivity, microbial and endotoxins (4.1.1):
Monitoring frequency, maintenance and corrective actions were specified. Methods of sampling and analysis were described in Appendix. For microbiological monitoring, R2A medium is recommended, incubated during 7–14 days at a temperature of 17–23°C.
The dialysate quality assurance process involves all dialysis staff members and requires strict protocols. The physician in charge of haemodialysis has the ultimate responsibility for dialysate quality.
All suggestions and questions about this Guideline are welcome to www.senefro.org.
La Sociedad Española de Nefrología elaboró en 2004 una Guía de Gestión de Calidad del Líquido de Diálisis. La segunda edición revisada de la guía ha tenido en cuenta nuevas evidencias y la normativa internacional. En la guía se hacen algunas recomendaciones sobre normas para preparar el líquido de diálisis: agua, concentrados y sistemas de dosificación de la hemodiálisis. Esta guía se basa en la norma ISO 13959, la Farmacopea Europea, la Real Farmacopea Española, las normas y prácticas recomendadas de la AAMI, la Guía Europea de Buena Práctica en Hemodiálisis, revisiones de la bibliografía, según su nivel de evidencia, y la opinión del grupo español de expertos.
Se definieron 2 niveles de calidad del agua: agua purificada y agua purificada de alta calidad (ultra pura), y para el líquido de diálisis: líquido de diálisis ultra puro. El uso habitual de líquido de diálisis ultra puro se recomienda en todo tipo de hemodiálisis para prevenir y retrasar la aparición de complicaciones: inflamación, desnutrición, anemia y amiloidosis.
Los requisitos de la calidad del agua, de los concentrados y del líquido de diálisis se definen como los niveles máximos admisibles de contaminantes: sustancias químicas (4.1.2), conductividad, microbiana y endotoxinas (4.1.1):
Se especificaron la frecuencia de control, el mantenimiento y las medidas correctivas. Los métodos de muestreo y análisis se describieron en los anexos. Para el control microbiológico es recomendable el medio de cultivo R2A, incubado durante 7–14días a una temperatura de 17–23°C.
El proceso de garantía de la calidad del líquido de diálisis implica a todos los miembros del personal de diálisis y exige protocolos estrictos. El médico a cargo de la hemodiálisis tiene la responsabilidad final de la calidad del líquido de diálisis.
Pueden dirigir sus sugerencias y preguntas acerca de esta guía a www.senefro.org.
Dialysate or dialysis fluid (DF) is one of the basic elements of haemodialysis (HD). It is a liquid medium which comes into contact with the blood through the semipermeable membrane in the dialyser during haemodialysis. It allows the exchange of substances, essentially solutes, with the blood, in both directions.
It is an electrolyte solution prepared extemporaneously by the haemodialysis machine (HDM) from purified water and solutes provided in the form of electrolyte concentrates or undissolved salts. Prepared in this way, dialysate is virtually isotonic and its electrolyte composition is similar to plasma. There are differences in the concentrations depending on the gradients necessary to achieve the appropriate balance of each substance according to the needs of the patient.
Dialysate quality and purity are two of the most critical requirements of haemodialysis. The presence of contaminants in the dialysate exposes the patient to the risk of toxic substances accumulating and consequent acute and chronic complications. Some contaminants can interact with cells or proteins, triggering bio-incompatibility on top of that caused by other components of the extracorporeal blood circuit in haemodialysis.
Dialysate quality and purity are the result of a complex series of processes in which any error has a huge impact on the final product. It is therefore necessary to monitor all the elements and steps involved in the entire production chain. The preparation, distribution and storage conditions must be designed to minimise the risk of chemical and microbiological contamination.
For ease of understanding, this guideline consists of six key subject areas:
- 1.
Water treatment systems (Section 4 and Appendix A.2.1).
- 2.
Electrolyte concentrates and powdered salts (Section 5 and Appendix A.2.2).
- 3.
Haemodialysis machine (Section 6.3 and Appendix A.2.3).
- 4.
Quality control (Section 7 and Appendices 3 and 5).
- 5.
Methods for correction and prevention (Section 8 and Appendix 4).
- 6.
Dialysate quality management (Section 9).
The guide includes: a glossary of terminology with references to the corresponding sections (Section 2); a quick guide with the standards (in bold) and key recommendations, divided into six sections (Sections 4–9); Section 10 with the rationale and evidence supporting the recommendations (Section 10); and Appendices 1–6 describing equipment components and methodology.
Introduction to the 2015 editionThe purpose of this first revision of the Sociedad Española de Nefrología (SEN) [Spanish Society of Nephrology] Guideline for Dialysate Quality (GDQ), published in Nephrology 2004;24 Suppl. 2:1–42, is to update it and bring it into line with haemodialysis today.
It is 11 years since the 1st edition of the GDQ and haemodialysis has evolved:
- 1.
Most haemodialysis (HD) in our area is high-flux (80–90%) and the proportion of patients on on-line haemodiafiltration (OL-HDF) has increased significantly (20–30%). Today's HD requires ultrapure dialysate.
- 2.
HD water treatment technology has also evolved. The effective treatment systems available these days for the water used in HD maintain fairly uniform standards.
- 3.
Although many of the HD water treatment systems in Spain have been renewed and improved, contamination above the specified limits continues to occur; mainly microbiological or involving aluminium and chloramines. Prevention of contamination must therefore be a goal.
- 4.
Maintaining the standard of highly purified water continues to create technical problems in many HD units. Certain requirements for highly purified water need to be refined or modified without affecting the requisite quality.
- 5.
Aspects relating to dialysate quality not covered in the first edition need to be included, such as feed water supply characteristics, testing methods for certain contaminants, portable and home water treatment systems and monitoring of central concentrate delivery systems.
The GDQ has set the standard to be followed in terms of HD water and dialysate quality in Spain and other countries. Many of the Spanish Autonomous Regions have used it for their technical specifications and agreements for HD. The Ministry of Health, Social Policy and Equality included it as a reference in its Standards and Recommendations for extrarenal blood purification (haemodialysis) units in 2011. The GDQ helped create a culture of awareness about the importance clinically of ultrapure dialysate, which was subsequently endorsed by a large volume of scientific evidence. It has been pioneering in a number of ways, one being that the microbiological testing methodology proposed in the 2004 GDQ is similar to that of the 2014 ISO 13959.
We have attempted to make the terms of this guideline more readily understandable and interpretable. We have therefore standardised the terminology used, as described in Section 3.3.
The GDQ was commissioned to a group of experts by the SEN. This revision has been commissioned by the present SEN Board of Directors, chaired by Dr Maria Dolores del Pino, to a new group of experts.
2Glossary of terms and definitions- AAMI:
Association for the Advancement of Medical Instrumentation: Recommends standards for medical procedures in the United States of America. www.aami.org. See Appendix 6.
- Action level:
Degree of contamination at which implementation of corrective measures is recommended to avoid reaching unacceptable contamination limits. See Section 4.1.1.
- Activated charcoal filter:
Filter used to remove chlorine, chloramines and organic substances from water by means of adsorption onto the microporous structure of the activated charcoal. See Appendix A.2.1.
- Actual volume of a bed:
The volume of a bed in a container. The space between the particles is considered as volume occupied by the bed itself. See Appendix 2.
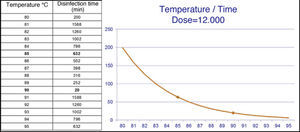
- Ao:
Ao is a way of calculating the “dose of thermal energy necessary” to disinfect, based on different combinations of time and temperature. One Ao equals one second at 80°C. (1 Ao=1second at 80°C). Ao=∑10 (T−80)/z·Δt, where T is temperature in °C; z equals 10°C; and Δt is the time in seconds. See Section 8.
- Backwash:
Process for a bed filter consisting of introducing water from the bottom to flow upwards to expand the bed and allow removal of retained particles. To be effective, the water velocity should be slightly greater than the fluidisation velocity to achieve expansion of the bed by at least 10%. See Appendix 2.
- Bed expansion:
Increase in the bulk volume of a bed when subjected to a backwash cycle. See Appendix 2.
- Bed filter:
Filter consisting of a container filled with a rigid material of uniformly sized granules that retains particles in the free spaces. Backwashes are necessary to remove retained particles. See Appendix 2.
- Biofilm:
Colonies of bacteria settled on the surfaces of hydraulic circuits, protected by an ecosystem of precipitated minerals and an extracellular mucopolysaccharide matrix, which reproduce and generate in stagnant conditions. The presence of biofilm is associated with persistent bacterial contamination. It is an active source of endotoxins and other biologically active bacterial derivatives. It is resistant to the majority of disinfectants. See Appendix 3.
- Buffer tank:
Tank installed at the beginning of a water treatment plant to provide a constant flow rate. Its function is not to store water, but to stabilise the process and not depend on the feed water supply pressure. See Appendix 2.
- Bulk volume of a bed:
The volume of a bed when expanded with a backwash cycle. See Appendix 2.
- Cartridge filter:
Consists of a cylinder of porous material which retains particles smaller than the pore size when water is passed through it. See Appendix 2.
- Chemosynthetic bacteria:
Bacteria capable of synthesising their nutrients and obtaining energy from inorganic compounds. See Appendix 2.
- Chloramines:
Products formed by the combination of free chlorine and ammonium. The ammonium can come from decomposition of vegetable matter and other organic pollutants, or be added by those responsible for the water's potability in order to disinfect it. Chloramines are extremely oxidative and toxic for patients on haemodialysis. See Appendix 5.
- Colony-forming units (CFU):
Unit of measurement of viable bacteria. Refers to the number of bacterial colonies which have developed in a solid culture medium. Expressed as CFU per millilitre of fluid. See Appendix 3.
- Combined chlorine:
Chlorine chemically bound to other compounds, like chloramines. Total chlorine equals free plus combined chlorine. See Appendix 5.
- Concentrates for dialysis:
Concentrates or salts to be mixed with the purified or highly purified water in the haemodialysis machine to form the dialysate. These concentrates or salts are manufactured, packaged and labelled to CE marking standards and must comply with ISO 13958. See Section 5.
- Conductivity:
Measure of a material ability to conduct an electric current and is the inverse of resistivity. The concentration of electrolytes in water is directly related to the electrical conductivity of the solution. It is measured in Scm−1. See Section 10.8.
- Continuous electric deionization (CDI) or electro-deionization (EDI) system:
Device for reducing the concentration of free ions in the water, cations and anions, applying an electric field. See Appendix A.2.1.
- Deioniser (DI):
Device for reducing the free ions in the water, with dual-bed or mixed-bed cationic and anionic resins. See Appendix 2.
- Dialysate:
Aqueous fluid containing electrolytes, buffers and usually glucose, which is formed by combining water and dialysis concentrates and salts in the dialysis machine. The terms dialysis fluid, dialysis solution or dialysis bath are synonymous. See Section 6.
- Dialyser:
Haemodialysis machine in which the dialysis takes place by way of diffusive and convective transport and adsorption. Inside, the blood and dialysate come into contact through a semipermeable membrane. Also called a filter.
- Disinfection:
Process for destruction of microorganisms, which reduces their numbers but does not eliminate them. Sterilisation reduces the numbers to a safe level, since total elimination is virtually impossible. Can be done by chemical or thermal means. See Section 8.
- Electro-medical equipment:
Active non-implantable medical device (ANIMD) and as such, subject to European and national regulations (i.e. RD1591/2009, Medical Devices Directive, 93/42/CEE, ISO13485, IEC 60601.1:2015). See Appendix 6.
- Empty Bed Contact Time (EBCT):
Contact time between the water and the activated charcoal bed. Calculated by the equation EBCT=(7.48*V)/Q, where V is the bulk volume of the bed and Q the water flow expressed in gallons/min. See Appendix A.2.1.
- Endotoxin units per ml (EU/ml):
Units of endotoxin (ET) quantified by a test based on Limulus amoebocyte lysate (LAL) activation. ET activity varies according to its composition, so its activity is compared to the E. coli standard (O 113: H10). The relationship between the activity and the mass varies with the batch of LAL and batch of ET standard. Generally, 0.012 endotoxin units are equivalent to approximately one picogram. The ratio is generally 10 EU per ng. Chromogenic determination is the most sensitive, although other methods (colorimetric, fluorimetric, GEL-CLOT) are routinely used in these determinations. See Appendix A.3.2.
- Endotoxin:
Biologically active pyrogenic substance, lipopolysaccharide, released from the outer cell wall of gram-negative bacteria. They are measured in Endotoxin Units, EU/ml, or in International Units, IU/ml, which are now equivalent. See Appendix 3.
- Exotoxin:
Protein with pyrogenic activity secreted by microorganisms. See Appendix 3.
- Feed or raw water:
Feed water means the water to be treated, whether it comes from the municipal water supply, is taken from a well or is delivered by tankers. In general it is potable water, which in Spain is subject to the relevant regulations. See Appendices 1 and 5.
- Fluidisation velocity:
The velocity of backwashing of a bed filter to which the filter is subjected to an upward force equal to its weight. Its bulk volume does not vary; expansion is zero. See Appendix 2.
- Forward rinse:
Process for a filter bed consisting of introducing water at the top and removing the water used in the backwash, which has not been filtered. See Appendix 2.
- Free chlorine:
Dissolved molecular chlorine. See Appendix 5.
- Haemodiafiltration:
Form of haemodialysis in which, alongside diffusive transport, convective transport plays an important role in solute removal.
- Haemodialysis machine:
Machine or system that carries out the haemodialysis process. The dialysis process takes place in a dialyser where the blood circuit and the hydraulic circuit combine to produce the dialysate. These two circuits are controlled by the machine with maximum efficacy and safety for the patient.
- Haemodialysis:
Form of renal replacement therapy that involves the removal of solutes from the blood and exchange of solutes between the blood and the dialysate. The solutes are preferentially removed by diffusion.
- Heterotrophic bacteria:
Bacteria, which metabolically, require organic compounds to develop. This is a broad and diverse group that includes symbiotic, saprophytic and pathogenic species. The term heterotrophic is commonly used as a generic name for water bacteria with few nutritional requirements. See Appendix 3.
- ISO:
International Standardization Organization. See Appendix 6.
- Limulus Amoebocyte Lysate (LAL) test:
Specific detection assay for endotoxins, based on horseshoe crab, Limulus polyphemus, amoebocyte lysate. See Appendix 3.
- Lipopolysaccharides (LPS):
Endotoxins composed of lipids and sugars (polysaccharides). See Appendix 3.
- Microfilter:
Filter able to remove particles larger than 1μm in diameter (0.1–0.3μm according to the ISO). See Appendix 2.
- Nanofiltration:
Retains organic compounds with molecular weights between 300 and 1000 D, retains some salts and works at lower pressure than RO. See Appendix 2.
- Nominal flow rate:
The flow rate produced by reverse osmosis equipment under ideal conditions. See Appendix 2.
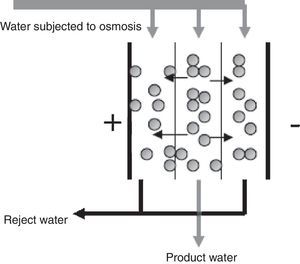
- Permeate or “filtrate”:
Fluid that has passed through a reverse osmosis membrane. See Appendix A.2.1.
- Pleated cartridge filter:
Cartridge filter consisting of a rigid perforated core in which the porous material is very thin and has a large surface area, “a kind of paper” folded in zigzag formation, sealed at both ends and attached to the core. The filtering capacity is determined by the porosity of the filter material. Can retain particles and bacteria up to 0.2μm. See Appendix 2.
- Pre-filter or “sediment or sand filter”:
Bed filter that removes large particles, from 500 to 20μm, positioned at the point of entry of the water into the treatment. Allows backwashing. See Appendix A.2.1.
- Pre-treated water:
Water that has undergone all the processes before reaching the osmosis or treatment equipment. See Appendix 2.
- Purified water:
Water for the preparation of drugs or dialysis fluids which do not necessarily need to be sterile or pyrogen-free. See Section 4.1 of this guideline.
- Pyrogen:
Substance that causes fever or inflammation. External pyrogens (endotoxins/exotoxins, bacterial DNA) induce cytokines such as IL-6, IL-1 or TNFα, which are mediators in the induction of fever and inflammation. Substances capable of activating mononuclear blood cells. See Appendix 3.
- Pyrogen-free sterile water:
Water free of live organisms and spores. Sterility is defined as the presence of fewer than 1×10−6CFU/ml viable bacteria or <0.03EU/ml. See Appendix 3.
- R2A:
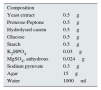
Culture medium for bacteria which, due to its high sensitivity, is particularly indicated for water contaminants. For composition see Appendix A.3.1.
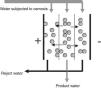
- Reject or “concentrate” water:
Water that has not passed through the osmosis membranes and contains practically all the salts and contaminants. See Appendix 2.
- Resin:
Cations, anions or mixture attached to granules in ion exchange beds, such as in water softeners and deionisers. See Appendix 2.
- Resistivity:
Resistance of a material to the flow of electricity through it. It is the inverse of conductivity. The fewer the electrolytes, the greater the resistivity. Resistivity of 1MΩ/cm is the same as conductivity of 1μS/cm. See Section 10.8.
- Reverse osmosis (RO):
Water purification process which works by sieving through a membrane and rejection of ion concentrate. Removes ions and organic contaminants with molecular weight >100 D. See Appendix A.2.1.
- SDI:
Silt Density Index. Parameter that measures the density of sediments or the fouling tendency of water. Measurement of this index is regulated by ASTM International (formerly American Society for Testing and Materials). See Section 10.5.
- Substitution fluid:
Fluid or dialysate used in haemofiltration and haemodiafiltration and infused into the blood circuit to replace the ultrafiltrate. It is mandatory for this to be ultrapure dialysate and undergo a second ultrafiltration for endotoxins. It can also be used for priming and flushing the blood circuit, as bolus, as infusion during the session or in blood return. Also called infusion fluid. See Section 6.
- TDS:
Total dissolved solids. Sum of all dissolved ions. Related to the electrical conductivity and used to test the effectiveness of reverse osmosis. See Section 10.8.
- TGEA:
Culture medium for bacteria, recommended by the ISO and the European Guidelines, along with R2A. Composition: Tryptone glucose yeast extract agar. See Appendix A.3.1.
- TSA:
Culture medium for bacteria. For composition see Appendix A.3.1.
- Ultrafilter:
Membrane filter (polysulfone, polyamide, polyethersulfone, posidyne) used to remove microbial components from dialysis water, post-treatment in the dialysis water or, most commonly, in dialysis fluids. Some ultrafilters retain ET by adsorption. Also used as a synonym of dialyser. See Appendix 2.
- Ultrafiltration, as dialysis method:
Convective transport of solutes across a membrane driven by a hydrostatic pressure gradient (transmembrane pressure).
- Ultrafiltration, as treatment of dialysate:
Process similar to RO. Rejects contaminants between 1000 D and 0.1μm. Ultrafiltration requires low pressures to operate. Essentially retains organic substances, bacteria and pyrogens. The effectiveness of ultrafiltration membranes is determined as the lowest molecular weight that rejects more than 90% (Molecular Weight Cut-off [MWCO]). See Appendix 2.
- Ultrapure dialysate:
Dialysate, preferably produced with highly purified water, with fewer than 0.1CFU/ml and fewer than 0.03EU/ml which has passed through an ultrafilter immediately pre-dialyser. See Section 6.
- Ultrapure water:
Ultrapure or highly purified water is defined as water meeting the recommendations on content in chemical contaminants listed in Section 4.1.2. Maximum conductivity is 5μScm−1, measured at 25°C, bacterial contamination is less than 0.1CFU/ml (10 CFU/100ml) and endotoxin levels must be less than 0.03EU/ml. See Section 4.2.
- Ultraviolet:
Bactericidal ultraviolet radiation used to kill microorganisms. UVC, wavelength-energy-photon: 200–290nm and 6.2–4.3eV. 254nm is recommended and 16 milliwatt-s/cm2 followed by use of an endotoxin filter. ISO 13958:2009. See Appendix 2.
- USP:
United States Pharmacopoeia. See Appendix 6.
- Venting:
Entry and exit of air that occur when the volume of a liquid stored in a rigid container varies. It may have a 0.2μm filter in order that the air entering meets specifications. See Appendix 2.
- Water softener:
Device for reducing water hardness by removing the calcium and magnesium by ion exchange with cations fixed on resins. See Appendix A.2.1.
- Wound cartridge filter:
A cartridge filter formed by a rigid perforated tube or core in which the porous material consists of a cord made of cotton, polypropylene or other similar material; depending on the type of yarn, the number of threads per revolution and the winding pressure, varying filtering capacity is obtained. Can retain particles from 1 to 100μm. See Appendix 2.
- AAMI:
Association for the Advancement of Medical Instrumentation: www.aami.org
- CDI:
Continuous electric deionization or electro-deionization system
- CK:
Cytokines or interleukins
- CSA:
Canadian Standards Association
- DI:
Deioniser
- EBCT:
Empty bed contact time; contact time with the activated charcoal bed
- EDI:
Continuous electric deionization or electro-deionization system
- HD:
Haemodialysis
- HDF:
Haemodiafiltration
- OL-HDF:
On-line haemodiafiltration
- HF:
Haemofiltration
- ISO:
International Organization for Standardization
- LAL:
Limulus Amoebocyte Lysate
- DF:
Dialysis fluid (dialysate)
- LPS:
Lipopolysaccharides/Endotoxins
- HDM:
HD machine
- RO:
Reverse osmosis
- ppm:
parts per million
- TMP:
Transmembrane pressure
- R2A:
Reasoner's 2A culture medium
- SDI:
Silt Density Index
- PS:
Pyrogenic substances
- LAL test:
Limulus Amoebocyte Lysate (LAL) test
- EBCT:
Empty Bed Contact Time (contact time with the activated charcoal bed)
- TDS:
Total dissolved solids
- TGEA:
Tryptone glucose yeast extract agar
- TSA:
Bacto Tryptic Soy Agar; Soybean-Casein Digest Agar
- EU:
Endotoxin Units
- IU:
International Units of endotoxins
- CFU:
colony-forming units
- USP:
United States Pharmacopoeia
The purpose of this guideline is to provide recommendations on quality for water, concentrates and dialysate in order to ensure that haemodialysis is effectively performed. The aim of effective haemodialysis is to promote optimal treatment for patients on haemodialysis. This guideline aims to provide unified criteria on the necessary quality of water, concentrates and dialysate, along with methods for achieving and maintaining that level of quality.
3.2ScopeThe scope includes all types of haemodialysis or haemodiafiltration or any extrarenal blood purification technique that uses dialysate. Geographically, the scope of application of this guideline is the Spanish National Health Service, as it is specifically adapted to the practice of haemodialysis in Spain today. It is intended to be seen as a goal for maintaining or achieving quality in Spanish haemodialysis units, and the hope is that its recommendations will be taken on board by all healthcare workers, dialysis technicians and companies engaged in the field of dialysis.
3.3MethodologyCreation of the expert group: The Spanish Society of Nephrology appointed a nephrologist with extensive experience in haemodialysis, and coordinator of the first edition of this guideline (2004), as coordinator of this second edition; the multidisciplinary working group was then set up, including four nephrologists and other specialists, experts in various technical and scientific aspects related with water treatment, a microbiologist, four haemodialysis technicians, a product manager and a renowned researcher in the area of biocompatibility in dialysis. The multidisciplinary approach is essential to provide a comprehensive picture of the subject, not only for the theoretical aspect of the literature review, but from the perspective of experience in day-to-day practice.
Dynamics of meetings: The first meeting was to decide on the aspects to be studied and divide them among the different experts. At subsequent meetings, each expert's proposals were discussed in an effort to reach a consensus on conclusions. A final face-to-face consensus meeting was then held.
An information search was carried out, covering literature (Medline/PubMed, Cochrane) other guidelines (see Appendix 6), reports by quality control agencies and technology assessments (ISO, European Pharmacopoeia, AAMI, etc.; see Appendix 6).
Analysis of the quality of the evidence: The grading system used was Grades of Recommendation Assessment, Development, and Evaluation (GRADE) (Uhlig K et al. Grading evidence and recommendations for clinical practice guidelines in nephrology. A position statement from kidney disease: improving global outcomes (KDIGO). Kidney Inter 2006;70:2058–2065). The quality (level) of evidence (A–D) and the strength of the recommendation (1–3) were arrived at by consensus at the proposal of the authors of each chapter. The quality of water, concentrates and dialysate can at times be a special case in terms of grading level of evidence. This area has been addressed in the ISO-AAMI. In some instances in this guideline, we have adhered to the latest editions of the ISO standards in order to meet one of the main objectives, which was to unify criteria with other recommendations/international guidelines.
The terminology used in this guideline has been standardised in relation to the level of evidence or the ISO standards as follows:
- –
“shall” means that compliance with a requirement or a test is mandatory (required=strong recommendation)
- –
“should” means that compliance with a requirement or a test is recommended, conditional on what is explained for that instance (recommended=weak recommendation)
- –
“may” is used to describe a permissible way to achieve compliance with a requirement or test (permissible or not graded)
As a basic rule, any treatment system for water for haemodialysis must be designed to, at the very least, meet the specifications for chemical and bacteriological levels recommended in this guideline, including in terms of maintenance of that system over time.
Water used for haemodialysis is divided into two distinct types: standard or purified water (4.1); and ultrapure or highly purified water (4.2). Each type has different criteria for microbiological quality and endotoxins. The current position of these guidelines is to recommend that highly purified water shall be used in haemodialysis units as the main component of ultrapure dialysate.
4.1Purified water for haemodialysis4.1.1MicrobiologyMaximum allowable levels for microbiological purityIn terms of bacteriological requirements, purified water used for diluting the dialysis concentrate shall contain fewer than 100CFU/ml. ISO 13959. 3rd edition 2014.
SpecificationsThese CFU figures correspond to the average of the total number of viable aerobic bacteria able to form a visible colony for each sample spread on R2A medium and incubated for 7 days between 17°C and 23°C (ISO 13959, 3rd edition 2014).
Although there is little literature base to support it, fungi should not exceed 10% of the total colonies of aerobic organisms (Evidence level C, 2).
For additional specifications and recommendations on this section, consult Appendix A.3.1.
Action levels for microbiological purityWe recommend that corrective measures, i.e. disinfection, be commenced when bacterial counts reach 50% of the mandatory limit: presence of more than 50CFU/ml of viable aerobic bacteria ISO 13959. 3rd edition 2014. Disinfection should also be performed with lower contamination levels if visible in more than one sample in order to prevent the formation of bacterial biofilm.
Maximum allowable levels of endotoxinsThe endotoxin content in purified water for haemodialysis shall not exceed 0.25EU/ml, measured by a sufficiently sensitive LAL test. ISO 13959. 3rd edition 2014.
Determination of the bacteriological quality of the water and the dialysate shall include testing for microorganisms and endotoxins.
Maximum allowable levels of chemical contaminantsPurified water for haemodialysis shall not contain contaminant concentrations greater than those listed below (ISO 13959:2014 [see Appendix 5]):
| – Aluminium: Atomic absorption spectrometry | 0.01mg/l (10μg/l) |
| – Antimony: Atomic absorption spectrometry | 0.006mg/l |
| – Arsenic: Atomic absorption spectrometry | 0.005mg/l |
| – Barium: Atomic absorption spectrometry | 0.100mg/l |
| – Beryllium: Atomic absorption spectrometry | 0.0004mg/l |
| – Cadmium: Atomic absorption spectrometry | 0.001mg/l |
| – Calcium: Atomic absorption spectrometry | 2mg/l |
| – Chromium: Atomic absorption spectrometry | 0.0140mg/l |
| – Copper: Atomic absorption spectrometry | 0.100mg/l |
| – Fluoride: Ion chromatography | 0.200mg/l |
| – Lead: Atomic absorption spectrometry | 0.005mg/l |
| – Magnesium: Atomic absorption spectrometry | 4mg/l |
| – Mercury: Atomic absorption spectrometry | 0.0002mg/l |
| – Nitrate, as N Colorimetry | 2.0000mg/l |
| – Potassium: Flame photometry | 8mg/l |
| – Selenium: Atomic absorption spectrometry | 0.0900mg/l |
| – Silver: Atomic absorption spectrometry | 0.005mg/l |
| – Sodium: Flame photometry | 70mg/l |
| – Sulfate: Turbidimetry | 100mg/l |
| – Thallium: Atomic absorption spectrometry | 0.0020mg/l |
| – Total chlorine: Colorimetry | 0.100mg/l |
| – Zinc: Atomic absorption spectrometry | 0.100mg/l |
All these elements shall be tested at least once a year. Testing for aluminium shall be six-monthly.
For more details regarding the analysis technique, please consult Table 3 of ISO 13959:2014.
Purified water shall have maximum conductivity of 5μScm−1at 25°C (Appendix 5). In exceptional situations, below 20μScm−1at 25°C shall be allowed while the cause of the increase in conductivity is being identified. (Level of Evidence C, 2)
If, despite having a water treatment system with double osmosis in series or osmosis plus an electro-deionization system in series, conductivity of 5μScm−1 is not reached, provided it is below 20μScm−1 and all chemical contaminants listed in Section 4.1.2 are within specified limits, the existing level of conductivity shall be set as reference. In such cases, the cause of the increase in conductivity (e.g. carbon dioxide, pH, Na, etc.) shall be identified.
Once the reference level is set, whether below 5μScm−1 or in the case of the above exception, in the event of significant increases in conductivity by more than 30% of the reference level, testing of all chemical contaminants and feed water quality and identification of the cause of the increase in conductivity shall be required.
The conductivity of the treated water shall be monitored daily, with the value, plus the reasons for any significant changes, being recorded.
The conductivity meter shall be calibrated at least once a year and we emphasise the utility of comparing it with the TDS.
The recommended maximum concentration of aluminium in treated water is 0.005ppm (5μg/l). (Level of Evidence C, 2)
4.2Highly purified water for haemodialysisHighly purified (ultrapure) water should be used to produce ultrapure dialysate for all types of haemodialysis. (Level of Evidence C, 1)
4.2.1Microbiology4.1.2Maximum allowable levels for microbiological purityIn terms of bacteriological requirements, highly purified water used for diluting the dialysis concentrate shall contain fewer than 10CFU/100ml (0.1CFU/ml). ISO 13959. 3rd edition 2014.
Specifications (see Appendix A.3.1)In order to measure these quantities accurately, it is necessary to analyse a sample of highly purified water greater than 100ml by filtration.
Action levels for microbiological purityWe recommend that action be taken when bacterial growth in cultures is visible with the presence of more than 5 CFU/100ml of viable aerobic bacteria. To increase the accuracy of colony counts, the volumes processed should be greater than 100ml. Cultures shall be repeated with a greater volume of sample and duplicate samples; if contamination is confirmed, disinfection should be performed to prevent the formation of bacterial biofilm.
Maximum allowable levels of endotoxinsThe endotoxin content in highly purified water for haemodialysis shall not exceed 0.03EU/ml, measured by a sufficiently sensitive LAL test. ISO 13959. 3rd edition 2014.
4.2.2Maximum allowable levels of chemical contaminants in highly purified waterHighly purified water for haemodialysis shall not contain chemical contaminant concentrations greater than those specified for purified water for haemodialysis (Section 4.1.2andAppendix 5).
Highly-purified water shall have maximum conductivity of 5μScm−1at 25°C (Appendix 5). In exceptional situations, below 20μScm−1at 25°C shall be allowed while the cause of the increase in conductivity is being identified. (Level of Evidence C, 2)
If, despite having a water treatment system with double osmosis in series or osmosis plus an electro-deionization system in series, conductivity of 5μScm−1 is not reached, provided it is below 20μScm−1 and all chemical contaminants listed in Section 4.1.2 are within specified limits, the existing level of conductivity shall be set as reference. In such cases, the cause of the increase in conductivity (e.g. carbon dioxide, pH, Na, etc.) shall be identified.
Once the reference level is set, whether below 5μScm−1 or in the case of the above exception, in the event of significant increases in conductivity by more than 30% of the reference level, testing of all chemical contaminants and feed water quality and identification of the cause of the increase in conductivity shall be required.
The conductivity of the treated water shall be monitored daily, with the value, plus the reasons for any significant changes, being recorded.
The conductivity meter shall be calibrated at least once a year and we emphasise the utility of comparing it with the TDS.
The recommended maximum concentration of aluminium in treated water is 0.005ppm (5μg/l). (Level of Evidence C, 2)
4.3Design of a water treatment systemThere is no one water treatment system generic to all dialysis units since much depends on: chemical and bacteriological quality of the feed water to be treated, its origin and possible variations in the elements dissolved in it over time; architectural constraints; quantitative needs; qualitative needs; financial budget; and prospective changes, both in terms of the water treatments themselves and new dialysis techniques.
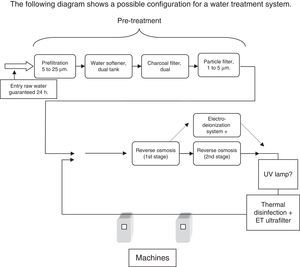
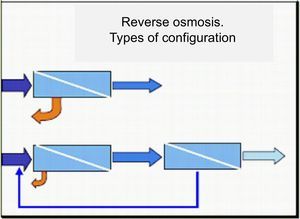
The basic components of a water treatment system for haemodialysis shall include a pre-treatment, where most of the undesirable elements are removed, and treatment with reverse osmosis and some other element that would achieve the level of purified water as part of its normal operation, generally a second stage of osmosis. (Level of Evidence C, 1)
Pre-treatment shall have at least one filter capable of retaining particles in suspension or sediment, water softener and charcoal filter (Appendix A.2.1) designed for the characteristics of the feed water supply, with duplicate equipment if levels of the element to be removed are considered high and could cause serious problems in the event of failure. (Level of Evidence C, 1)
It is essential to be aware of the potential problems at later stages as a result of poorly-designed pre-treatment; chlorine can damage the osmosis membranes or the presence of calcium can saturate them, or these elements can pass into the distribution network and so reach the patient.
The charcoal filter shall always be installed immediately pre-RO, as close as possible, as once the water is dechlorinated, there is a serious risk of contamination, particularly when passing through other filters where the speed slows down. (Level of Evidence C, 1)
When the feed water contains high levels of chloramines and other organic contaminants, or municipal, industrial or agricultural water contamination, the use of two activated charcoal filters in series is recommended.
Osmosis membranes shall be installed post pre-treatment, interposing a filter of at least 5μm, to avoid the possibility of small charcoal particles passing through, this being an essential element of treatment to obtain water that meets the quality requirements set out in the cited standards.
Installation of other elements post osmosis stage guarantees better water quality. Such elements may consist of: a second stage of osmosis, fed by the permeate from the first, with independent pumps between the two stages so that, in the event of failure of one, the other can continue to supply water; or an electro-deionization system. Resin deionization systems are not recommended due to the high risk of contamination (see Appendix A.2.1).
Both electro-deionization systems and UV lamps should always have ultrafilters installed which are capable of retaining up to the endotoxin concentration limit; electro-deionization systems have no filtering capacity and the bactericidal action of the UV lamps can add endotoxins to the water.
The working tank prior to the RO shall be as small as possible. The elements post first osmosis stage shall be installed in such a way as to allow different configurations, so they may be added to or complemented; a second stage of osmosis is recommended as the best option.
Elements which can be disinfected and/or descaled shall have the capacity to have accessories fitted to allow this function to be performed as quickly and reliably as possible: incorporated disinfectant metering pumps, programmed wash systems, built-in programmes in the equipment itself and sampling points.
4.4Water storage and distributionOnce treated, the water shall be distributed directly to points of use without storage tanks or drums, with the surplus being returned to the treatment entry point. The piping and plumbing shall be designed to prevent bacterial contamination and be easily disinfected. (Level of Evidence C, 1)
StorageTreated water shall not be stored as this makes it liable to contamination. Storing water creates difficulties for disinfection.
As there are no tanks of treated water, supply of feed water must be guaranteed, and the systems may therefore be as follows:
- –
Double water supply.
- –
Feed water tank, which shall have the characteristics described below and in Appendix 2 (A.2.1.1).
- –
Pre-treated water tank, with the same characteristics as the above point. In the case of the pre-treated water tank, some type of preservative or disinfectant treatment is required to guarantee that the water does not become contaminated.
If water tanks do exist, regardless of the volume, they shall be hermetically sealed, opaque, preferably stainless steel and have a conical base with the water outlet at the bottom and a 0.2μm antibacterial vent filter. The water inlet must be in shower form.
The volume of water required to complete a day's operation of the haemodialysis unit shall be guaranteed.
Distribution networkTreated water is highly inclined to acquire substances from the elements it comes into contact with, so the distribution network shall be made with materials that do not leach anything into the water, or are not suspected of being able to do so (copper, iron or aluminium pipes cannot be used), shall have no dead legs, using continuous piping with no joints or intersections, and be as short as possible. If stainless steel is used it must be pharmaceutical grade. The pipe that feeds the machine from the distribution network should be considered as an integral element of the distribution network. It has to circulate at a speed that minimises the risk of contamination and biofilm formation, >1m/s, so the section has to be precisely calculated. Unused water shall return to the water treatment system and go through it again.
Joins in the plastic materials form recesses and mean sudden changes in the linearity of the pipe that lead to reservoirs and disruption of the laminar flow; plastic materials which do not exhibit these drawbacks are now available on the market. Such connections are found in elbow joints when installed to change the direction of the pipe and in diversions to machines and valves. When opting for a particular type of material, consideration has to be given to how the connections are made, whether welded or using adhesives, due to the possibility of adhesives breaking down over time and leaching undesirable elements into the water. Polymer pipes and tubes are now available which do not have these drawbacks and resist heat without deforming. This type of material should be used for the distribution network (see Appendix 2). If the option is stainless steel, it has the advantages that thermal or chemical disinfection systems can be used and that it is resistant to accidental impacts and traction. The type of welding on stainless steel piping is crucial to prevent subsequent corroding of welded joints.
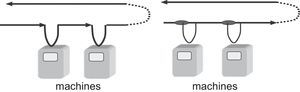
Dead legs must be completely avoided; outlets to machines shall be considered as dead legs and therefore shall also be avoided, with the emphasis on those where the pipes are translucent. The distribution network therefore has to go to the machine; one way to do this would be by installing a U-bend, where the distribution network goes to the machine and returns, then goes on to the next machine; this has the disadvantage that the pipe leading to the machine has the same section as the rest of the network.
The other way to do it is with secondary rings: a primary circuit distributes water throughout the unit and a secondary circuit carries the water to the machine; obviously, the dimensions of this secondary circuit are smaller than the primary; in the event of breakage or blockage, only the machine connected to it would be affected.
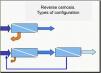
The above figures show the different configurations to ensure constant flow of water to the machine: U-bend installation on the left and secondary rings on the right.
Endotoxin retentive filterThe distribution loop for haemodialysis water shall be fitted with an endotoxin filter when any of the following three conditions exist: there is storage of treated water; double-pass osmosis is not available; post UV lamp, if the quality level to be achieved is of ultrapure water. The endotoxin filter shall preferentially be fitted at the beginning of the loop.
Thermal disinfection systemThermal disinfection systems are highly recommended and should at least be installed for the treated-water distribution loop. In conjunction with the secondary rings and the disinfection methods, combined with that of the haemodialysis machines, they are the most effective way of preventing the development of biofilm in the haemodialysis water distribution loop. Moreover, they avoid the risk of contamination of the dialysate by chemical disinfectants.
The following diagram shows a possible configuration for a water treatment system.
4.5Water treatment systems in special and mobile units and for home dialysisHaemodialysis water treatment systems in Special Units, such as high-dependency, ICU, resuscitation, etc. shall have the same characteristics as those described above and have the capacity to produce purified water for dialysis, as specified in this guideline, while being subject to the same controls and preventive measures as the other haemodialysis water treatment systems.
Water treatment systems for home dialysis and mobile dialysis units shall at the very least be fitted with an activated charcoal filter, reverse osmosis and a 0.2μ bacteria filter. The water quality shall be monitored monthly, as specified in Section 7 of this guideline, and the system shall undergo preventive measures as described in Section 8. Once it has been verified in an initial biochemical analysis that the substances referred to in Section 4.1.2 are at allowable concentrations, the conductivity of the osmosis permeate (or product) water shall be recorded and taken as reference. Sudden and significant changes in conductivity shall be investigated. After periods of inactivity, if the water treatment system is to be used again, the same methodology shall be applied.
Home haemodialysis machines shall come with their own endotoxin ultrafilter, which shall be replaced according to manufacturer's instructions.
5Concentrates for dialysisThe solute supply systems for the production of dialysate may be individual, for a single haemodialysis machine or central, for a group of machines.
The water used to produce concentrate for dialysis shall at the very least comply with the standards required for purified water specified inSections 4.1.1and4.1.2. (Level of Evidence C, 1)
Ideally, it should have the level of quality required for water for injection.
Currently, only bicarbonate shall be used as basic concentrate.
Its composition should be adjusted according to the clinical situation of each patient, as is done with other factors that influence the efficacy and safety of haemodialysis.
The concentration of solutes identified on the labelling shall be present to within a margin of ±5% or 0.1mEq/l, with the exception of sodium and chloride where the margin of variability shall be ±2.5%. These margins are expressed relative to the concentration in the dialysate after dilution of the concentrates. ISO 13958: 2009.
All the components shall be stated on the labelling along with their quantities and level of purity. The dilution to be used shall be stated as parts of concentrate per parts in the final solution (dialysate). The labelling shall state the expiry date, which guarantees its stability.
If the concentrates contain non-traditional components, the margin of tolerance for such components shall be ±5% of the nominal concentration of that component.
We recommend that manufacturers should provide chemical and microbiological quality certificates for the batches of concentrates supplied.
The containers, including caps and stoppers, shall be made of materials that do not interact with the concentrate, thereby contaminating it, and shall be airtight.
5.1Individual concentratesAcid concentrateAn acidic solution of concentrated salts which may contain dextrose. It is diluted with purified water and the bicarbonate concentrate to produce dialysate. In general, most patients can be dialysed with standardised ion concentrations in acid concentrate, although the type of concentrate should be individualised for each patient. (Level of Evidence B, 1)
Bicarbonate concentrateA concentrated solution of sodium bicarbonate which is diluted with purified water and the acid concentrate to produce dialysate. Some bicarbonate concentrates also contain sodium chloride. (Level of Evidence C, 1)
Current recommendations are that bicarbonate in powder form should be used for producing dialysate. (Level of Evidence C, 1)
Surplus bicarbonate from dialysis shall be discarded. (see Appendix A.2.2). (Level of Evidence C, 2)
Bicarbonate and citrate concentratesThe acid concentrate usually contains acetic acid as stabiliser for the mixture with bicarbonate. It is used at concentrations of 3–10mmol/l. Such concentrations cause transfer of acetate to the patient during HD, raising blood acetate concentrations. Exposure to acetate is increased in on-line haemodiafiltration techniques. The increase in blood acetate levels has been associated with a number of undesirable effects in the patient and there have been efforts for some considerable time now to find other acids to use as stabilising agents for dialysate. Citrate dialysate is a new alternative to using acetate as acidifying agent. Citrate is a calcium-chelating agent that is also used for its anticoagulant effect as it reduces calcium ion concentrations. A number of long-term beneficial effects have been described in relation to citrate, including reduced thrombogenicity, improvement in clearance, inflammation, nutrition and tolerance, and improved control of acid-base balance leading to less pre-dialysis acidosis. More scientific evidence is required to justify switching from acetate to citrate in all haemodialysis.Citrate concentrates suitable for different machines are currently available on the market, without acetate or with a mixture of the two stabilising agents.
Forms of presentationAcid concentrate comes in:
- •
Container
- •
Bag
- •
Dry cartridge of sodium chloride+bag of ions
- •
Bulk delivered concentrate (see Section 5.2)
Under this classification, we have presentations that facilitate individualisation of treatment, others that do not and those with which individualisation is impossible. The bulk system makes individualising treatment difficult or even impossible in terms of the concentration of solutes in the dialysate.
The dry cartridges of sodium chloride plus ion bag fully facilitate individualisation without increasing the storage space required; since they are single-use, they are also very safe.
The containers of concentrate allow individualisation, but an increased number of formulas have to be stored, meaning more space is required, and they can be less safe.
Bags allow individualisation, take up less space and are preferable in terms of safety since they are single-use and cannot be refilled.
Bicarbonate concentrate comes in dry cartridges or containers:
We recommend that containers of bicarbonate shall no longer be used, replacing them with bicarbonate powder cartridges. (Level of Evidence C, 2)
5.2Central concentrate systemsIndividual concentrate systems are preferable to central concentrate systems from the point of view of safety and individualisation, but they are more expensive and create greater problems in terms of storage and waste. The central production systems for bicarbonate concentrate are the most liable to microbiological contamination and are therefore not recommended. (Level of Evidence C, 1)
In situ central concentrate generator system shall be designed to include a source of purified water, easy draining and grounding for electrostatic discharge. They shall be made with materials that do not cause contamination to water, are non-corrosive and prevent the formation of mould and algae.
Tanks used to store the mixture shall be emptied and cleaned of debris and residue before using other concentrate baths in order to prevent cross-contamination between the different concentrate formulas. Use of additives in the acid is prohibited, as they can distort the composition of the dialysate; only potassium or calcium may be added and this shall always be indicated on the labelling, stating the final concentration.
Sodium bicarbonate tanks are not recommended but, if used, in addition to meeting the above characteristics, the walls and bottom shall be cleaned and disinfected. Sodium bicarbonate may be used in liquid or powder form. In both cases, but particularly with powder, the concentration shall be monitored prior to distribution; generally between 34mEq/l and 40.8mEq/l.
The other mode consists of bulk delivered concentrate. They come in large containers or cisterns and are then delivered directly to the dialysis machine. The installation shall allow sufficient space and adequate access for that purpose.
The distribution network for concentrates shall be colour-coded, with the outside of the pipes painted red for those delivering acid mixture and blue for sodium bicarbonate. We also recommend that the tanks should be translucent so that levels are always visible; level-indicator tubes should not be used, especially not in the case of bicarbonate, due to the risk of bacterial growth.
The acid concentrate distribution systems and, if applicable, the preparation tank, must have programmes for disinfection, descaling and cleaning of the installation, and this shall be done at regular intervals, at least annually, to guarantee optimal microbiological quality of the system. See Section 8.2.
5.3MicrobiologyThe maximum allowable levels for microbiological contamination shall be identical to the purity level for purified water at the end of valid shelf-life. Microbiological contamination levels for concentrates are the same as for purified water (seeSection 4.1). (Level of Evidence C, 2)
Once opened, containers of individual bicarbonate concentrate shall be handled with care to prevent further bacterial contamination. Previously-opened containers shall not be re-used; any fraction left over from a dialysis session shall be discarded. (Level of Evidence C, 2)
Concentrates which have undergone some type of sterilisation or disinfection procedure shall be preferred.
5.4Chemical contaminantsThe grade of purity of the elements used for preparation of the concentrates shall be high and comply with the corresponding standards. Purified water shall be used to prepare concentrates. (Level of Evidence C, 1)
The manufacturer should specify the requirements regarding chemical contaminant concentrations for purified water. In the United States, Chemical Grades are regulated by the USP/National Formulary. The salts used in the preparation of the concentrates can be a source of contamination and metal poisoning for the patient (see also ISO 13958: 2009).
6Dialysate qualityUltrapure dialysate qualityWe recommend that ultrapure dialysate shall be used for all types of haemodialysis, haemodiafiltration and haemofiltration.
6.1Maximum levels of microbiological contamination in dialysateMaximum allowable contamination in ultrapure dialysate is 0.1CFU/ml for bacteria and 0.03EU/ml for endotoxins. ISO 1163: 2009.
The testing method is the same as for highly purified water for haemodialysis.
To minimise inflammation in patients on haemodialysis, all dialysis units shall use ultrapure dialysate for all types of haemodialysis. The routine use of ultrapure dialysate requires the fitting of specific ultrafilters in the dialysate circuit. (Level of Evidence B, 1) The ultrafilters shall be used and replaced according to the manufacturer's instructions.There shall be a protocol for action when dialysate contamination is demonstrated in a haemodialysis machine. The action measures shall include withdrawal of the machine, replacement of the ET ultrafilter and general revision of the machine's operation. Full disinfection and descaling shall be carried out, including the point of connection to the loop, the tubing, the internal hydraulic circuit and the connectors.
6.2Maximum concentrations of chemical contaminantsThe specifications are the same as for purified and highly purified water (Sections 4.1.2 and 4.2respectively), except for the solutes used for the concentrates and the resulting conductivity. (Level of Evidence B, 1)
6.3Dialysate preparationA haemodialysis machine is a medical device designed to mix the concentrated electrolyte solutions or powder with the treated water to an electrolyte concentration, pH and temperature determined by medical prescription. The dialysate water shall be suitably degassed. The amount of electrolytes diluted in the water is monitored by dual systems, by measuring electrical conductivity and/or the pH of the final solution (pH meter). The temperature is monitored by thermometer. Before being delivered to the dialyser, the dialysate has to have passed through at least one ultrafilter. Hydraulic circuits in the machines, with no dead spaces, are preferred. A machine's entire hydraulic circuit has to have an automated and programmable disinfection system.
The conductivity and composition of the dialysate shall be exactly as prescribed by the doctor.
Regular use of ultrapure dialysate over the long-term helps prevent or delay a number of the complications associated with haemodialysis. (Level of evidence A, 1)
7Quality controlThe chemical and microbiological purity of water and HD fluid shall be monitored regularly and the results recorded. There shall be protocols in place with details of the procedures to be followed in the event that the performance limits or prescribed limits are exceeded. These protocols shall even contemplate the temporary closure of the dialysis unit when breach of the required safety limits reaches unacceptable levels. (Level of evidence C, 1)
7.1Technical monitoring of the process componentsDaily monitoring of key elements or parameters, which is easy and quick to oversee, helps ensure the proper operation of the various components of the water treatment system; it can also prevent an emerging problem in one component from developing and causing serious repercussions on some other part of the system, and ultimately affecting the quality of the treated water. Both feed water and treated water shall therefore be tested daily for the following: Free and total chlorine (chloramine) levels; hardness; and conductivity. At the same time, the pressures and flow rates in the different components of the water treatment and distribution equipment shall be monitored. (Level of Evidence C, 1)
Current mandatory requirements regarding the quality of water for haemodialysis mean that all the elements involved in producing this water need to be more strictly monitored. It is essential to keep an accurate record of all tests and procedures performed on the water treatment system and to follow the prescribed maintenance protocols for each element in the system. A protocol shall be drawn up in advance detailing the procedures to be followed in the event that faults are detected. The necessary procedures will depend on the particular water treatment system, the staff involved, characteristics of the unit, etc. so the protocol shall be written individually for each haemodialysis unit.
The technical and user manuals must be available for the different items of equipment. The staff responsible for monitoring shall receive sufficient training and information on all elements of the water treatment system and have the appropriate accreditation.
Routine testing can vary depending on the equipment and the quality of the water to be treated; in some cases it may be necessary to perform tests more frequently, especially in the validation or commissioning phase. In this phase, testing should be done daily, at least at the start of dialysis sessions and on completion of the last of the day, to check the effectiveness of the filter elements, both to verify the correct volume of the these elements and the programming of regeneration and/or backwash steps.
The frequency of monitoring of the water treatment system is based on two levels, for both technical and analytical monitoring: the first level, during the validation of a new treatment plant, refurbishment of an old facility or after a contamination requiring corrective action; the second level, during maintenance, day-to-day management of the treatment system, after the end of the validation period. The tests to be performed can be classified into three areas: technical; chemical; and microbiological.
The following table is intended as an aid to organising the tests to be performed. It should be used to complement the testing instructions provided by the equipment manufacturers; although, given the context in which their equipment is used, they can at times be a bit “relaxed” about the frequency.
| Element | Daily testing | Monthly testing and procedures | Comments |
|---|---|---|---|
| Flow rates and pressures (pressure gauges) | Check throughout the course of the treatment for any abnormal variations in pressure. | Certain automatic actions of the treatment system, essentially self-cleaning, cause variations in the normal pressures. | |
| Entry of feed water | Pressure | Measure chlorine – chloramines, hardness and conductivity. | Increase testing frequency if changes in conditions are suspected: drought, irrigation in the proximity, work on cisterns, etc. Any change can affect elements of the treatment system or the final quality and make some type of modification necessary. Compliance with Royal Decrees 140/2003 and 865/2003 |
| Prefiltration (sand, iron-retention filters, etc.) | Pressure difference between input/output and state of the programmer. | If filters are auto-washable, verify operation of the wash cycle. | The operation or condition of subsequent elements may indicate the proper functioning of the prefilter. Make changes to the filter element following the manufacturer's or installer's instructions. |
| Water softener | Test hardness at output and record, indicating the softener active at that time and the remaining volume for the next regeneration. State of the salt (brine) tank. | Check consumption of salt, the various stages of regeneration, condition and operation of the control elements: flow meters; programmer; etc. | Alterations in conductivity pre-osmosis and decreased reject and permeate flow rates may be indicative of faults in the water softeners. Do not prolong the life of the resins beyond that recommended by the manufacturer. There are specific devices for monitoring hardness on-line. |
| Charcoal filter (dechlorinator) | Measure free and total chlorine (chloramines) at the output of each charcoal filter at maximum consumption. Should be done once per shift if there are no treated water tanks. If there are treated water tanks, also measure post tanks. | Check operation of the wash cycle - expansion. Condition of automatic control elements. Test subsequent filter. | Replace charcoal at least once a year. If there are two charcoal filters in series or parallel, it shall be possible to perform measurements independently. The condition of the subsequent filter is indicative of the operation of the charcoal filters. |
| Microfiltration prior to reverse osmosis equipment | Pressure difference between inlet and outlet. | SDI test. Microbiological testing pre-RO and/or disinfection, from the dechlorinator outlet to the feed inlet to the RO equipment, if repeated contamination or malfunction of the RO is detected. Depending on the results, the frequency can be extended. | This element, from the dechlorinator outlet to the RO equipment, is susceptible to growth of microorganisms of various kinds in the absence of chlorine; this can have a major impact on the final microbiological quality of the water, hence the need for prevention and/or monitoring. |
| Reverse osmosis equipment | Conductivity at inlet and outlet or total dissolved solids (TDS). Pressures and flow rates. Ion rejection. Check programmer (alarm history, overview). | Check operation of automatic actions not visible in usual operation such as night operation, auto-rinsing of membranes, etc. and equipment control and protection elements. | Perform disinfection and descaling of the membrane according to manufacturer's specifications. Respect flow rates and pressures specified by manufacturer; in the event of unexpected variations, a detailed analysis (chemical, bacteriological, endotoxins, SDI) should be carried out of elements pre and post RO equipment. |
| Element | Daily testing | Monthly or other frequency of testing and procedures | Comments |
|---|---|---|---|
| Electro-deionization system | Conductivity or resistance, pH. | Verify operation alarm and measurement systems. | Increase in conductivity (or reduction in resistance) or change in the pH suggests saturation or malfunction of some element, resulting in high risk of contamination. The alarm should be adjusted to very low levels to allow the defect to be corrected (≈1 Mohms/cm=1μS) |
| Ultrafilters | Inlet and outlet pressure, input, outlet and rejection flow rates. | Exclusive microbiological and endotoxin analysis should be carried out at the feed inlet and outlet to check for effectiveness, regardless of the analyses performed in the rest of the treatment system. | High risk of loss of pressure due to clogging. Respect the maximum useful life stated by the manufacturer (time or clogging). Whenever replacing, carry out disinfection of the corresponding part of the hydraulic circuit. Rupture carries the risk of massive release of retained elements, e.g. endotoxins. |
| Ultraviolet (UV) lamp | Light intensity 16–30 milliwatt-s/cm2 | Change the lamp according to the manufacturer's technical specifications. | |
| Distribution network (including water intake haemodialysis machines) | Check pressure at the inlet and outlet of the distribution loop. Circulate water through dead legs if any. Measure chlorine/chloramines when there are treated-water tanks. | Monitor disinfectant rinses when used. | Set disinfection schedule based on the characteristics and length of the network, product water quality, type of disinfection (thermal, chemical). Every disinfection shall be recorded along with the reasons (protocol or contamination). The outlet to a non-working machine shall be considered a dead leg. |
| Tanks | If for treated water, measure chlorine/chloramine levels and hardness in distribution network. | Switch drive pumps (automatic systems). Check operation of levels and alarms. | If for treated water, disinfect with the distribution network. Change vent filter according to specifications. If for raw or pretreated water, chlorine/chloramine levels have to be monitored to prevent contamination. Check for presence of silt and general cleanliness. |
| Haemodialysis machines | Machines in reserve shall be disinfected at least once a week and rotated periodically. Strictly comply with the manufacturer's instructions for disinfection, maintenance and monitoring, especially those relating to ultrafilters inserted in the dialysate delivery to the dialyser. Check that the waste pipes from the machines do not have physical contact with the piping they drain into (Floating drain or contactless drain); clean these last one” regularly to avoid deposit of substances from dialysis. Each machine shall have a maintenance and incidents record. | Haemodialysis machines, in effect, act as dead legs and can alter water quality and therefore that of the dialysate and, in extreme, cases, affect the entire unit. In specific cases (e.g. repeated contamination), microbiological monitoring of the drains may be necessary; be aware that these points are easily contaminated from the external environment, and results must therefore be interpreted based on that premise. | |
Monitoring of the water system should be performed at different points of the dialysate production process, at different frequencies according to the circumstances:
Validation period of a new water treatment system following installation or a major refurbishment, or after having detected high levels of contamination which required corrective action. What exactly constitutes a major refurbishment or corrective action can be difficult to define; the following scenarios should be taken as a guide:
- –
Any change affecting designation as “Medical Device” of any of the equipment, according to Royal Decree 1591/2009.
- –
Changes in feed water quality resulting in installation or modification of any part of the pre-treatment and/or osmosis equipment.
- –
Repeated contaminations which, in addition to the appropriate disinfection, require modification of any part of the installation due to being identified as possible source of the problem.
The validation period for a water treatment system refurbishment can be compatible with the operation of the haemodialysis unit. After the refurbishment, a full disinfection shall be carried out and the proper operation of all the elements of the water treatment system and that the conductivity of the treated water is <5μS/cm−1 shall be verified. At the same time, the analytical tests specified in this section shall be performed.
- –
Maintenance period: Maintenance of a system in routine operation.
Microbiological monitoring of purified and highly purified water shall be done weekly for the first month after commissioning of the unit (validation phase). If any of the cultures or ET are positive, the necessary corrective measures shall be taken and the validation period extended for a further month. Subsequently, and during the maintenance phase, monitoring shall be carried out at least once a month. (Level of Evidence C, 2)
Testing for endotoxin levels shall be performed monthly in both the validation and maintenance periods. (Level of Evidence C, 2)
Each centre shall establish a written protocol setting the frequency, method and responsibilities for these tests. Operational units shall test the quality of the dialysis water at least monthly, using the culture methodology and sampling points specified in this guideline. For more details, see Appendix A.3.1.
Sampling points for microbiological cultures: In the validation period, samples shall be collected of the following: feed water; softened water; the water immediately pre-RO equipment; the treated water immediately post-RO; water from the end of the distribution loop and from at least 10% of the water intakes for the machines (HD stations); and the dialysate on entry into the dialyser in a minimum of 10% of the machines.
Microbiological testing of the water shall not be performed within the first 24hours following disinfection.
In the maintenance period, samples of the treated water shall be collected for cultures and ET from the first point and last point of the loop, post osmosis and at 10% of the connection points to the machines. After 12 months, samples shall have been taken from connection points to all the machines, including unused connection points. Samples of dialysis fluid shall be taken for cultures and ET pre-dialyser in 10% of the machines. After 12 months, all machines, including reserves, shall have been tested. (Level of Evidence C, 2)
In the maintenance period, it is not necessary to take samples of feed water or samples from intermediate points of pre-treatment, unless contamination is detected or alteration in the quality of the treated water suspected.
Sampling should be carried out post charcoal filter and pre reverse osmosis equipment, especially if there is no specific protocol for regular disinfection of this section.
For machines, the same criteria shall be followed as in the validation period, such that all machines shall undergo microbiological testing at least every 12 months.
In facilities with storage tanks for treated water, samples shall also be taken pre and post tank, generally coinciding with the outlet from the osmosis equipment and the beginning of the distribution loop respectively.
In the event of environmental microbiological contamination of the haemodialysis unit, additional samples may be taken of the drainage from the machines, but it is important to be aware that this section of piping is exposed to external contamination, because of its proximity to or direct contact with the drainage system.
In home haemodialysis with production of dialysate, microbiological monitoring should be done monthly, possibly alternating a water sample with a dialysate sample.
There are local regulations and guidelines which are stricter in terms of number of samples and testing frequency.
7.2.2ChemicalConductivity, corrected to 25°C, shall be measured continuously in the permeate from the osmosis. The reading shall be visible and connected to an alarm system to alert any changes.
Water hardness, free chlorine and total chlorine (chloramines) tests shall be performed daily. The sampling points are specified inSection 7.1. They should preferably be performed prior to the first haemodialysis session of the day.
Testing for all chemical contaminants specified inSection 5.2(maximum levels of chemical contaminants) shall be performed twice in the validation period and annually in the maintenance phase. Aluminium shall be monitored every six months.
The sample for the determination of chemical contaminants shall be obtained from one of the water connection points to the machines.
Water hardness shall be tested daily using a titration method or permanently with an alarmed instrument. Regeneration shall be adapted to the cycle of volume, salt activity, capacity of the resins and the water hardness; the programmer status shall be checked daily.
If the disinfection system for potable water uses chloramines, concentrations in the water shall be determined indirectly by measuring the free and total chlorine and calculating the difference.
There are local regulations and guidelines which are stricter in terms of number of samples and testing frequency.
8Methods for correction and prevention8.1Methods for correction and prevention for waterThe procedures for disinfection, descaling and detergent action are an integral part of the maintenance system for the water plant and distribution network. The frequency, type of disinfection and descaling (heat, chemical, mixed) and periodic changes in their components (filters, resins, membranes, ultraviolet lamps) shall be according to the manufacturers’ instructions and based on the results of the microbiological tests. (Level of Evidence C, 1)
The aim of maintenance of a dialysis-water treatment system and dialysis machines is to prevent contamination, not treat it. Methodology based on treating contamination is not acceptable. Prevention is based on scheduled disinfections, with frequency and type depending on the design of the water treatment system and the dialysis machines. Analytical tests are used to verify proper operation, not to indicate when disinfection is necessary. (Level of Evidence C, 1)
Automated disinfection systems should be used in the treated-water distribution circuit; they may operate by heat or chemicals, or be mixed, and should include an endotoxin filter. Automated systems make it easier and safer to maintain microbiological standards. Frequent automatic programming helps prevent contamination. We recommend programmable thermal disinfection of the distribution loop for maintenance of highly purified water production. (Level of Evidence C, 1)
It is important that the construction materials for the circuit do not contribute to chemical contamination of the water (aluminium, zinc, copper, etc.) and are compatible with the different disinfectants to be used in maintenance. The most suitable materials for a water distribution circuit are: stainless steel (at least SS316); acrylonitrile butadiene styrene; cross-linked polyethylene (PEX-A); polypropylene; polyvinyl fluoride and polyvinyl chloride and new materials such as PVDF (polyvinylidene fluoride). Whichever the case, they shall be labelled for sanitary use and CE marked. Materials resistant to high temperatures should always be used as preference.
Stainless steel allows thermal and chemical disinfection, but it is important to use an approved steel grade and also that the welded joints are not subject to corrosion.
Preventive disinfection can affect the tanks, pre-treatment, treatment, loop and machines.
Cleaning of the haemodialysis room and drains is another aspect to be considered.
In view of the above, outlines of preventive disinfection and corrective disinfection are provided below. Minimum preventive disinfection would be as follows:
Disinfection of the loop; shall be done at least every four months. If microbiological contamination is detected, corrective disinfection shall be performed and the frequency of the preventive disinfections increased.
In the case of an automatic heat disinfection system, recommended to produce highly purified water, this shall be programmed to run at least once or twice a week.
Full disinfection, which includes pre-treatment, shall be done at least annually, to coincide with replacement of the activated charcoal.
Ideally, the disinfection of the loop should be done in conjunction with disinfection of the machines.
When there are reservoir tanks of treated water, disinfection of the loop shall include the tanks.
According to the requirements of the ISO standard (ISO_DIS 23500), the circuit of the reverse osmosis unit shall be disinfected regularly. This includes:
- •
The reverse osmosis membrane and all the internal components of the RO circuit
- •
The distribution pipes
- •
The post-RO tanks and filters
The individual concentrates shall meet the labelling specifications. If they are found to be contaminated, they shall be rejected and exchanged for a non-contaminated batch and the incident reported.
In central systems, the descaling and disinfection and other forms of prevention and treatment shall be done as specified by the supplier. We recommend that disinfection shall be carried out at least every 12 months and bacteriological analysis tests performed 3 days prior to disinfection.
If microbial contamination of dialysate is detected while levels in water are in the acceptable range, the concentrate shall be suspected as source of contamination and the appropriate cultures performed.
8.3Corrective actions for dialysateMaintenance and periodic disinfection of the haemodialysis machines are mandatory for preventing proliferation of bacteria and biofilm formation in the hydraulic circuit. In order to prevent bacterial contamination and the transmission of viral diseases, we recommend that they shall be disinfected after each haemodialysis session. (Level of Evidence B, 1)
Before commencing any haemodialysis session, it is mandatory that the composition of the dialysate is exact and that any disinfectant has been completely rinsed out.
The type of disinfection and the regimen shall be as recommended by the manufacturer of the machine. They shall be approved by the senior clinician in charge of the renal unit and all staff involved in the process informed. Disinfection and rinsing shall be recorded along with the name of the person responsible.
The machine tubing and all elements in the hydraulic circuit which are not covered by the automatic disinfection of the machine shall be disinfected at least annually.
Once disinfection of any element of the dialysate production, water treatment, centralised concentrate preparation and dialysis machines is completed, appropriate rinsing shall be performed followed by testing for residual disinfectant. SeeAppendix 4.
9Dialysate quality management9.1StaffThe success of the water and dialysate quality management process lies in the collaboration of all staff working in the dialysis unit (nephrologists, nurses, technicians, analysts, microbiologists and prevention specialists) and adherence to procedures; all those involved shall strictly follow the established protocols.
There shall be at least one person responsible for quality management of the water treatment system.
The responsibility may fall to the person in charge of the maintenance and servicing of the water treatment unit. If this is contracted externally, it shall be carried out jointly together with a designated senior member of the haemodialysis unit staff. The staff responsible for quality management should be specifically trained in the use of the water treatment equipment, the proper methodology for the tests and corrective actions.
The staff responsible shall be subject to periodic audits to confirm their competency.
The procedures shall include the temporary closure of the dialysis unit if contaminant safety limits are exceeded.
9.2Resources requiredThe dialysate quality control protocol shall be properly specified and followed by those responsible. The means for proper operation shall be as specified in each of the sections in this guideline. The specified human and material resources shall be provided by the company or the public administrative body responsible for the care of the patients on haemodialysis. The company or institution owner of the haemodialysis unit shall be responsible for providing the means necessary for proper operation.
Dialysis shall not be performed without water filtered by activated charcoal and subjected to osmosis.
9.3DocumentationThere shall be a paged record book or secure, controlled-access computerised form where all actions taken concerning water treatment shall be recorded, as specified in this guideline. The person in charge of the water treatment system shall be responsible for completing it.
The results on the chemical and bacteriological purity of the dialysis water shall be monitored periodically and regularly, and those results shall be duly recorded. Well-documented procedures shall be in place informing of the steps to be followed in the event that the limits are exceeded. Corrective actions shall also be recorded.
9.4ResponsibilitiesEach person involved in managing the production of dialysate is responsible for their own duties.
Ultimate responsibility for the dialysis fluid being as per specifications, both in terms of chemical composition and microbial contamination, and for it meeting the standards described herein, is the senior clinician in charge or the head of the dialysis unit.
The public body, public/private partnership or private company responsible for the healthcare of the patients on haemodialysis, shall ensure all necessary means to deliver this standard of quality.
The procedures shall include the temporary closure of the dialysis unit if contaminant safety limits are exceeded.
Over 96% of the 90–240litres per session of dialysis fluid (dialysate) that comes into contact with the patient through the dialyser is made up of haemodialysis (HD) water. Contaminants in the water can be transferred to the patient and lead to accumulation in large quantities. Added to that is the fact that renal failure prevents the patient from being able to eliminate accumulated contaminants, leading to actual poisoning. The medical literature contains many reports of acute and chronic poisoning in haemodialysis patients caused by water contamination and leading to significant morbidity and mortality.1–17 As water is the main component of dialysate and the most difficult to standardise, it is one whose production must be most closely controlled.
In the early days of haemodialysis as treatment technique for chronic kidney failure, the aim of water treatment was to prevent hard water syndrome and bacterial contamination.5 Later, difficult-to-remove contaminants had to be tackled, such as certain metals – aluminium poisoning for example causes encephalopathy and osteomalacia1,2 – or chloramines, which can cause outbreaks of anaemia in haemodialysis units due to haemolysis.3,8 Meanwhile, cases have continued to be reported of poisoning by different contaminants.10–16
More recently, the focus for concern has moved to an area bordering between clinical and subclinical repercussions which includes the problem of pyrogens/endotoxins. We now know that although not all our patients suffer pyrogenic reactions, many are exposed to endotoxins, leading to a chronic inflammatory state which, over time, negatively affects patients’ health in many different ways.17–28 Our present objective should be to achieve an ultrapure haemodialysis fluid which only contains water and the necessary components. Dialysate plays a key role in the biocompatibility of HD; hence the importance of its quality.18,21,26,27,29
However, it is difficult to determine where to establish the cut-off points for potentially toxic substances in dialysate. The AAMI sets the limits according to the toxicity of the particular substances.30 In the first category, they include the solutes added to the dialysate, such as Na, Ca, Mg and K and set levels that do not affect the final concentration in the dialysate. In the second category, they include substances regulated by the standards for potable water, such as arsenic, cadmium, lead, etc. (see Appendix 5), setting the limit at 10% of that. Included in the third category are substances with particular significance for patients on dialysis in terms of poisoning, such as chloramines or aluminium, setting the limits for their concentrations according to the values reported as toxic in the literature.30 Since 1981, new potential toxic substances have steadily been discovered, either from the water, components of the water treatment system, concentrates or machines, resulting in new elements having to be added to the list of those to be removed and monitored.31 More attention is also now given to bacterial contamination and the potential consequence of that, endotoxaemia. This guideline has taken as maximum limits those set by the ISO 2014, complemented on occasion by the European Pharmacopoeia standards. This is obviously a process under constant review, dependant on the discovery of new toxic substances or new levels of toxicity, and we should therefore highlight three possible changes for the future.
The first refers to aluminium concentrations. The aluminium in water is present as ions associated with salts and in colloidal form, bound to organic material. Depending on the pH, the ion form can vary from a trivalent cation to a complex anion. Softeners only remove the cation forms. Colloidal aluminium is not removed by deionisers (DI); the only process with the capacity to remove it is reverse osmosis (RO). Aluminium is sometimes added to water as flocculant for organic matter, so it can be in very high concentrations. In such situations, the only way to achieve optimal levels in the dialysate is to work in series with two RO.1
We know that the balance of aluminium during dialysis is established between free or ultrafiltrable plasma aluminium, 5–10% of the total, and the aluminium in the dialysate and, if we want to create a distinct negative balance, maintaining Al concentrations in blood below 30–50μg/l, we have to maintain the concentrations in dialysate below 5μg/l.32 These days, we want HD patients to maintain aluminium levels below 20μg/l.
Measuring substances such as aluminium requires a precise methodology, the use of non-metallic needles and special tubes, and preventing contamination of any kind. The procedure used is atomic absorption spectrophotometry, in graphite furnace to avoid contamination. In view of aluminium's special characteristics, if the aluminium concentrations in water are good, i.e. <5μg/l, and water conductivity is below 5μS/cm, we can presume that the water's ion properties are also acceptable and that the other anions and cations will also be as required. Possibly the exception to this rule is water with very high content in mercury or iron, in which case flocculation and chelation systems shall be required to remove these elements.
The second subject for discussion is the allowable concentration of chloramines. Chlorine is added to potable water as a bactericide because it is a strong oxidising agent. This function is performed by free chlorine, which quickly dissolves. The way to maintain stable levels of free chlorine is the formation of chloramines, mono-, di- and trichloride nitrogen compounds, which release the chlorine slowly. Chloramines are able to pass through most water treatment systems, including reverse osmosis.33,34 There are essentially two systems for removing them from water: reaction with activated charcoal; and reaction with sodium bisulfite. The choice of one system or another depends on the characteristics of the water to be treated and the pH these reactions cause as, depending on the type of membrane, they will affect the performance of the reverse osmosis system. For the production of purified water for HD, activated charcoal is recommended31,34,35 as it is easier to maintain, it is safer and it has a broader spectrum of retention, although proper maintenance and regular renewal is essential. If small amounts of chloramines cross into the blood, they cause oxidant effects, the most important being haemolysis. Chloramines are difficult to measure so concentrations are usually estimated as the difference between total chlorine and free chlorine, although this method lacks sensitivity. When measured using this method, the allowable levels should be less than 0.06mg/l for total chlorine and 0.05mg/l for chloramines, not 0.1ml/l as the limit currently stands.31,35,36 Published data35,37 show higher rates of anaemia associated with chloramine levels around 0.1ppm. An alternative solution which is used in North America is placing two activated charcoal filters in series and doing the determinations between the two.
The third point for discussion is how to test for pyrogenic substances; those which trigger inflammation in patients on haemodialysis. The standard method is the LAL test. Should we be using more sensitive tests to detect endotoxins (ET) or should we be looking for simple, standardised tests for ET and other pyrogenic substances? This subject will be discussed later.
The complexity and cost of the water treatment system therefore differs significantly according to the desired degree of purity. Two different grades of purity can be used for the water in haemodialysis: purified and highly purified (ultrapure). Purified water is the basic form of treated water, valid for conventional haemodialysis. Microbiological contamination of purified water must comply with the recommendations of the European Pharmacopoeia: bacterial count less than 100CFU/ml; and less than 0.25EU/ml. In addition to other requirements specified in these guidelines, highly purified water must contain less than 10 CFU/100ml and less than 0.03EU/ml.
Highly purified water is recommended for producing ultrapure dialysate. The dialysate's degree of purity will be that of the worst of its components. Ultrapure dialysate is recommended for all types of haemodialysis. In 2015 in Spain, 80–90% of haemodialysis was carried out with high-flux disposable dialysers. Ultrapure dialysate is also necessary for high-flux HD and in haemodiafiltration and haemofiltration with on-line production of substitution fluid.
Financial constraints affecting the ability to produce dialysate free of bacterial or ET contamination are the reason behind many guidelines continuing to allow two levels of quality. Optimally, dialysate should be of pharmaceutical quality, similar to that of parenteral solutions. We know that stimulation of monocytes can be observed with plasma ET levels as low as 0.05ng/ml.38 We have to bear in mind that the stimulation occurs with cumulative exposure during the HD session and is also enhanced by other adjuvant stimuli, such as complement, activated by the dialyser membrane, or the acetate in the dialysate. That is why we have chosen ET levels for ultrapure dialysate similar to those for sterile fluids, thereby ensuring that there are insufficient levels of ET to stimulate monocytes. Bacterial contamination is the source of ET and other substances with pyrogenic properties and should therefore be as low as possible. The limit for purified water of 100CFU/ml, as indicated by most standards for use, including the European Pharmacopoeia, may not be low enough. To obtain dialysate with low bacterial contamination, levels of contamination in its three components must be low. We also have to take into account the fact that dialysate is a better medium than water for bacterial growth and so the exponential growth rate of bacteria is higher in dialysate than in purified water. Despite these difficulties, we recommend that the microbiological quality of water for producing ultrapure dialysate shall be that of highly purified (ultrapure) water.39,40
Nowadays, the key component of a haemodialysis water treatment system is reverse osmosis (RO).
In technical terms, the design consists of the optimal configuration of the various components of the water treatment system as regards size, position and purity to ensure the quality of the treated water. The combination of a water pre-treatment system (softener, activated charcoal and microfilters), reverse osmosis modules, and a system of direct piping, with no storage tanks if possible, is the minimum configuration required to produce purified water and prevent microbiological contamination.
To produce highly purified (ultrapure) water, a system is required based on having a second reverse osmosis module and/or an electrochemical deioniser placed in series. A system such as this allows the production of highly purified water according to very demanding purity criteria.
To prevent bacterial contamination and biofilm formation, the water distribution system must be designed meticulously. Acceptable materials for the piping system are stainless steel and polyethylene. Major effort should be directed at achieving adequate configuration of the distribution loop, with straight pipes, favouring continuous flow at high speed and preventing water stagnation by avoiding dead spaces.1,2 Inclusion of heat disinfection in the water treatment system, with automatic programming, preferably synchronised with that of the machines, secondary loops and an ultrafilter for endotoxins are essential for obtaining highly purified water.3
Distillation is a water purification system based on the change in state from liquid to gas by heating, then condensing by cooling to return to its original liquid state. It is effective in removing all kinds of contaminant materials except volatile contaminants. Despite its great effectiveness, it is not commonly used in haemodialysis as it is expensive and cumbersome.1,2
See Appendix 2 for further information.
The concentrate can be where bacterial contamination of dialysate starts, especially if bicarbonate is used in liquid form, as it is an excellent medium for bacterial growth. Moreover, the salts used in the preparation of the concentrate can lead to heavy metal poisoning.1
The Spanish Royal Pharmacopoeia and the European Pharmacopoeia2 set out the characteristics that concentrates or solutions for haemodialysis must comply with. Final concentrations of Na are allowed a margin of ±2.5%, variability unlikely to be accepted in clinical practice. For Na, a variation of 2.5% represents ±3.5mmol/l, and so for a concentration of 140mmol/l, means a range from 136.5 to 143.5mmol/l. Haemodialysis with these two extreme concentrations is entirely different.3 Although through the control of conductivity, the machines can partially correct these errors, it also has its variability. The margins of variability for K, Cl, Mg and Ca are ±5%, but in these cases there is less clinical significance. The same standard establishes the allowable endotoxin concentration as less than 0.5EU/ml in the diluted solution, but does not specify the method of determination. As a method of detecting pyrogens, it proposes inoculation into rabbits. In this guideline, the level of microbiological quality has been set at the level required for purified water. The margin of variability has been set at ±2.5% for Na in accordance with ISO 13958:2009, and how to determine the ET shall be determined by LAL test, as it is better standardised.
The quality of concentrates is based on the degree of purity of its components, both water and solutes. Keshaviah et al.4 suggest that limits for contamination levels, essentially of trace metals, should at the most be of a magnitude that, once diluted to make the dialysate, do not exceed the levels specified for purified water, Section 4.1.2. The European Pharmacopoeia 3rd Edition determines that the aluminium content in sodium chloride used for haemodialysis concentrates shall be less than 0.2ppm.
Citrate dialysate is a new alternative to using acetate as acidifying agent. Citrate is a calcium-chelating agent that is also used for its anticoagulant effect as it reduces calcium ion concentrations.5 A number of long-term beneficial effects have been described in relation to citrate, including reduced thrombogenicity, improvement in clearance, inflammation, nutrition and tolerance, and improved control of acid-base balance leading to less pre-dialysis acidosis.5–13 More scientific evidence is required to justify switching from acetate to citrate in all haemodialysis.
Many technical aspects of haemodialysis machines have improved but it is still not possible to guarantee the sterility of the hydraulic circuit during operation. The dialysis bath barrels on the early machines were a point of bacterial contamination, as were the recirculation systems, the canisters. The closed loop ultrafiltration control systems also presented problems for disinfection. Today's machines represent a distinct advantage over the earlier models as far as disinfection is concerned. The use of bicarbonate as an alkalising agent has been a real problem in terms of the risk of bacterial contamination. None of the bicarbonate proportioning systems is sterile and contamination risk is very high. The risk of bacterial contamination of acid concentrates is minimal compared to bicarbonate.
Routine use of highly purified water to supply the HD machines is not enough to guarantee the microbiological purity of the dialysate. The bicarbonate in dialysate is an excellent culture medium for bacterial growth and can be the source of bacteraemia and pyrogenic reactions. The machine contributes to dialysate contamination because of the complexity of its hydraulic circuit. Both the design of the circuit and other factors such as inadequate disinfection encourage the growth of bacteria and biofilm formation in the circuit. Microbial contamination of the dialysate or the presence of by-products from the bacteria are potential causes of disease in HD patients and must be prevented through the use of highly purified water. As specified in Section 7, the quality of the dialysate shall therefore be tested regularly, verifying that the quality specifications in Section 6 are being met.
MicrobiologyMicroorganisms exist that are perfectly acclimatised to hostile environments, for instance, where there are virtually no nutrients, such as treated water circuits. They are special microorganisms and have to be treated as such. Dialysate bacteriological quality depends largely on the design of the water treatment plant and its distribution system, the quality of dialysis concentrates and disinfection of the circuit and machines. It also depends on the testing methods we use. We are referring in particular to the culture methods for water and dialysate samples.
EndotoxinsWith a closed system, microorganisms cannot pass from the dialysate into the blood, but ET and other pyrogenic substances (PS) can. PS are products excreted by the bacteria or by-products released upon bacterial cell lysis. Some of these substances have molecular weights of less than 10kD and can therefore cross the dialysis membranes by diffusion. The best known endotoxins are lipopolysaccharides (LPS), components of the outer membrane of gram-negative microorganisms, the majority of which can be detected by the LAL test. There are also other bacterial toxins (PS), such as exotoxins, peptidoglycans and muramyl peptides. A shared characteristic of these substances is their ability to act as pyrogens. Of all these contaminants, ET are the ones with the greatest pyrogenic potential.
The determination of bacterial ET is done with the limulus test (LAL), and may be performed using: gelation, perhaps the most common, based on the ability of these substances to form gels; turbidimetry, based on the ability of the endotoxins to react with endogenous substrates which are cleaved to generate turbidity; and colorimetry, a modification of turbidimetry based on the ability of endotoxins to react with chromogenic substances. In addition to the above quantitative methods, there are other methods for detecting ET contamination based on their immunogenic activity, such as the production of cytokines (CK) by monocytes or neutrophils, isotopic labelling and determination of anti-ET antibodies. As we have said, the method most commonly used is the LAL test. For testing the purified water, a simple technique such as Gel-Clot shall suffice. However, if it needs to be sufficiently sensitive, i.e. 0.01EU/ml, kinetic chromogenic tests shall be required.1
Factors such as the composition or the degree of turbidity of the vehicle for the sample can interfere with ET determination, so different standard samples should be sent to the laboratory to serve as reference. Also, ET easily adhere to certain plastics, so glass or low-affinity plastic tubes for proteins (Mini-Sorp tubes) should be used.
The ET detectable by LAL only account for a part of the total potential PS and they are also the ones with the highest molecular weight. The most useful method for determining PS in haemodialysis is to measure the impact on patients through cytokine production by monocytes, but this method is both laborious and expensive. A simpler method has been proposed.2 PS pass into the blood in the dialyser mainly by back-filtration, but those with low molecular weight can also pass by back-diffusion. Passing into blood has been demonstrated with all dialysis membranes and it does not only depend on the quantity of PS, but also on their quality. Pyrogenic reactions are more common with high-flux than low-flux membranes.3 PS are able to cross the high-flux membranes more easily and back-filtration is also more common with these. In testing for ET, it is not only the method that is important, but also the time the samples are taken and how they are stored. Once the PS are in the blood, induction of monocytes is not linear. Other factors come into play which can increase or decrease the production of CK.4 The process is multifactorial and is influenced by the quantity and type of toxin, the type of membrane, plasma factors and the concomitant action of other monocyte activation and inactivation systems.5 It is known that the presence of proteins, or whole blood, is a potentiating factor. At least two proteins are known to be necessary in this process: lipopolysaccharide-binding protein (LBP), a transporter protein; and bactericidal/permeability-increasing (BPI).6 Certain counter-regulatory CKs, such as IL-10, also play a part,7 as do nutritional and immune system status. For monocytes to produce CK, the simultaneous presence of other stimuli or signals, such as complement activation by the dialyser membrane or acetate in the dialysate, is very important.
This all explains why there is generally such poor correlation between colony-forming units (CFU/ml), ET levels detectable by LAL and CK production. Destroying bacteria decreases their concentration in the fluid, but may lead to an increase in ET concentrations, and the ET are capable of inducing the CK production if they pass through the dialysis membrane and are joined by other concomitant monocyte-activating factors. Some ET at plasma levels as low as 0.05ng/ml are able to induce the formation of IL-1.8
Prolonged activation of CK results in a series of alterations to the immune response, causing a state of chronic inflammation.9–15 Pyrogenic reactions occur in 1–5% of haemodialysis sessions. They occur more frequently with high-flux membranes and decrease if the dialysate is filtered through a membrane with adsorbent capacity, such as polysulfone or polyamide.16–18 Polysulfone, polyamide or posidyne ultrafilters are effective for filtering the dialysate and deliver a fluid with negligible levels of endotoxins and bacteria. This means less CK production.16,19,20 These filters achieve their effect not only due to their cut-off, which is 60kD and therefore higher than the molecular weight (MW) of many PS, but also by adsorption. It is therefore important that they be regularly replaced.
A proportion of the endotoxaemia that occurs in haemodialysis patients and some of their inflammatory, vascular and nutritional complications are related to exposure to endotoxins and other pyrogenic substances in dialysate.20
Dialysate is produced in the dialysis machine; the age of the machine, regardless of how well-maintained, is related to the safety and efficiency of that process. We recommend that haemodialysis machines shall have a useful life not exceeding seven years or 30,000hours of operation.21,22
The frequency of monitoring of the water treatment system is based on two levels, for both technical and analytical monitoring: the first level, during the validation of a new treatment plant, refurbishment of an old facility or after a contamination requiring corrective action; the second level, during maintenance, day-to-day management of the treatment system, after the end of the validation period. The tests to be performed can be classified into three areas: technical; chemical; and microbiological.1–4
Technical checks on the production of purified water include:
Check that the feed water to be treated meets water for human consumption quality criteria.5–7 It is essential to have information on the contaminants and changes occurring in the feed water, and any changes must be detected at an early stage to prevent collapse of the water treatment system and massive poisoning. A significant proportion of incidents related to water for haemodialysis, or to the operation of the treatment system, stem from the quality of the feed water.8–12
The proper performance of the water softener and dechlorinator and status of the programmers that operate the running-regeneration and/or backwash system shall be checked daily; we recommend before starting the daily sessions, but it is also useful at the end of the operating cycle, before regeneration, and even combining the two, primarily in the validation phase. The hardness of the water leaving the water softener shall be tested. Disposable titration kits may be used of <1mg/dl sensitivity; either a permanent measuring system using an automatic probe equipped with an alarm or colorimetric methods. The water softener regeneration system depends on the amount of resin and brine and the programming of the control valve. For dechlorination, total, combined and free chlorine shall be tested using disposable kits, reagents or by installing a chlorine meter in the circuit post activated charcoal filter(s), in order to monitor chloramine levels and their removal.13–15
The proper operation of the reverse osmosis and/or electro-deionization system shall be checked daily by measuring the conductivity of the permeate, the percentage of reject water and working pressures.16–19
Regular monitoring of SDI (primarily in the water pre reverse osmosis system, but also in the feed water or throughout the pretreatment) provides information on the quality of the water the RO equipment is having to work with and is particularly important when the life of the membranes is considerably shorter than expected and/or when the desired water quality is not being achieved. The conditions of the water to be treated can vary substantially over time; increases or changes in solvents (especially the colloidal type) may be such that they end up not being retained in adequate proportions by pretreatment. Having this information helps determine possible actions to be undertaken to optimise the working conditions of the RO membranes: modifications or corrective actions in the water pretreatment; requesting information on the feed water quality and the conditions of hydraulic systems prior to the water treatment system, etc. The SDI pre RO shall be at least<5 and the lower it is, the better the performance and longer the life of the RO membranes.20–22 The reference value for the required SDI can be found in the technical sheets provided by the different manufacturers of RO membranes and in any of the manuals on water treatments for haemodialysis units.
The regularity with which the SDI is measured shall be determined by the actual conditions and the variables described. There will even be units where it is not necessary to determine the SDI regularly because of the good quality of the raw water.
The details for chemical monitoring are discussed in Appendix 5. This should always be approached with the premise of avoiding chronic poisoning.
In terms of microbiological contamination, the first priority is to prevent the dialysate from being a source of bacteraemia and pyrogenic reactions.23–25 The second, more difficult to achieve, is to prevent ET and other pyrogenic substances passing to patients, and this means regular monitoring. When repeated positive cultures over 50CFU/ml are observed and are resistant to disinfection, biofilm and dead spaces in the piping have to be suspected.26,27 Weekly monitoring for the first month during the validation phase is mandatory. During the maintenance phase, monitoring shall be done at least monthly. Microbiological monitoring is an integral part of this guarantee process. There shall be protocols for documenting the degree of contamination of the water along the entire length of the chain. There shall be sample collection devices placed at key points to provide adequate levels of monitoring. Water samples shall be cultured regularly as specified in the section on microbiological monitoring, looking for the highest sensitivity.28,29 We recommend R2A culture medium and, in certain cases, TGEA in these guidelines.30
The frequency and methods used for microbiological analysis shall be tailored to the degree of contamination of the plant in particular and the frequency of disinfection of the system. The methods used for microbiological monitoring are described in Appendix 3. The most sensitive method shall be used, although adjusted to the degree of contamination. Microbiological monitoring shall be done with particular emphasis on critical parts of the chain, the most important being the end of the treated water distribution loop.
Endotoxin levels shall be monitored at least monthly. We recommend that the LAL test shall be used, ensuring that it has sufficient sensitivity for the measurement to be made.31,32
Documentation in graph form and storage of all physical, microbiological and chemical tests is critical throughout the monitoring process, so that data can be used as an assessment tool over time and as accreditation of achieving and maintaining the quality of both the components of the process and the products obtained.
Lastly, it is important to remember that both haemodialysis machines and water treatment systems come under the rules governing medical devices.33
Although no general rules can be laid down, it is a fact that frequent disinfection of the water treatment system is critical for preventing contamination. When a new treatment system is put into operation, it shall be disinfected weekly in order to adequately clean the resins and the piping system. The frequency of disinfection shall then be adapted to the configuration of the system in question and the results of the microbiological tests. The optimal interval between disinfections shall be established based on the kinetics of recontamination after each disinfection process. The only way to prevent biofilm formation is the advance use of the appropriate disinfection method. The frequency, type of disinfection, concentration and exposure time to the agent shall depend on the type of material used in the circuit and shall conform to the manufacturer's recommendations. We recommend full disinfection of the entire system at least once a year.
Standard operating procedures shall be in place for regular disinfection of the water treatment system to prevent the formation of biofilm. The maintenance of the water treatment plant shall include a series of measures involving frequent disinfection cycles, whether chemical, heat or mixed, of the entire chain, filter and exchange resins, which shall depend on the degree of contamination, and destruction of biofilm in the circuit. Periodic replacement of the various system components, such as resins, water softener and electro-deionization system, activated charcoal and filters shall be carried out according to the microbiological results and the expiration date. This avoids spread from highly contaminated resins.1
One major problem is the formation of a bacterial biofilm in the circuits. This is generally associated with repeated counts of more than 50CFU/ml in water or dialysate. In order to destroy biofilm, it is essential to use both disinfectants and detergents at sufficient concentrations and with sufficient exposure time.2,3 In some instances, it may be necessary to inspect the installation and even change components.
HD machines shall be disinfected after the end of each session, whether by heat or use of chemical agents. Proper maintenance of the machines involves regular cleaning of the hydraulic circuit with a detergent that removes organic residue, descaling with an acid solution to remove calcium and phosphate precipitates and chemical and/or heat disinfection. In all cases, cleaning, descaling and disinfection shall conform to the manufacturer's recommendations. The circuit should be replaced if found to be highly contaminated or biofilm is detected.
Cleaning of the water treatment system, distribution and haemodialysis machines shall generally be performed in line with the specifications of each manufacturer, which shall be according to the corrosion resistance of the materials used. There may be occasions when, despite following these specifications, we find bacterial contamination resistant to the treatment. Should this occur, we have to change product; first determining its properties and mode of action. The cleaning has to achieve three goals: (1) bacterial, including spores, fungal and viral disinfection; (2) descaling; and (3) cleaning or removing deposits of proteins, lipids and other organic products by detergent action. The three actions are interlaced. An example is the treatment of a bacterial biofilm, where cleaning and descaling is more important than the bactericidal action. Each of the main chemicals used in the cleaning process has a predominant action: hypochlorite is, in sufficient concentrations, a good bactericide and cleaner for organic deposits; peracetic acid is essentially bactericidal and has some descaling properties; acetic acid descales and is moderately bactericidal; and citric acid is the best agent for descaling. This means that achieving the three goals requires the use of more than one product, such as the classic combination of hypochlorite and acetic acid. When there is evidence of significant deposits of calcium and magnesium carbonate in the circuits of the machines, citric acid is required.
With the methods of disinfection, the contact or exposure time required for bactericidal action has to be taken into account. This can vary greatly according to the disinfectant and the microorganism to be treated and depending on the concentration achieved and the temperature; 4% formaldehyde at 20°C requires 24hours to achieve good disinfection, while 1% peracetic acid at 20°C needs 11hours and 0.75% glutaraldehyde only 1 hour. Another aspect is the ability of these substances to cause corrosion; sodium hypochlorite (bleach), which is a good detergent due to its oxidising properties, is able to modify the properties of certain membranes such as polysulfone, increasing removal of proteins 10-fold.4 Among the different disinfectant substances, incompatibilities exist, and they cannot therefore be used together; if necessary, they should be used sequentially, after suitable rinsing. Acetic, citric and peracetic acids should not be mixed with hypochlorite or hydrogen peroxide or with any of the bases in general. Aldehydes cannot be mixed with acids, ammonia or phenol. In general, all these substances are toxic and shall be handled with suitable precautions. The majority can trigger allergic reactions. There are a number of disinfectant mixtures available on the market specifically designed for haemodialysis: Instrunet HD® (1.15% sodium chlorite and 0.06% peroxyacetic acid); Dialox® (hydrogen peroxide, acetic acid and peroxyacetic acid) and Actril® (0.8% hydrogen peroxide and 0.06% peroxyacetic acid)5; Puristeril 340® (hydrogen peroxide and peroxyacetic acid); Cold Sterilant® (20–24% hydrogen peroxide, 4–6% peracetic acid and 8–10% acetic acid).
The methodology for the disinfection of the water treatment system shall cover the following aspects: disinfection shall be done periodically, before a high level of contamination is detected; the product or products used shall be selected according to the recommendations mentioned above and those specified by the manufacturer. The scheme mentioned below is designed for Dialox®, but can be used for other disinfectants changing the concentration and contact time. In this case, the concentration to be used is 5%; 5 litres of Dialox® diluted in 95 litres of water. This solution has to spread to and remain in contact with all points of the system for at least 30minutes. The contact shall preferably be under dynamic conditions, with the disinfectant circulating. A stringent wash-out shall then be carried out, followed by testing at various points, and in particular the connections to the machines, to confirm that the disinfectant has been rinsed away. Suitable detectors shall be used; in the case in question, potassium iodide starch paper, which detects up to 40ppm or enzymatic strips that detect up to 7ppm. Before disinfection it shall be confirmed that all the system components are compatible with the disinfectant.
Regular microbiological monitoring is essential to optimise disinfection and check its effectiveness.
In order for the dialysate and haemodialysis machines to meet basic safety standards, according to the machine type and technical specifications, there shall be a standard operating procedure with the step-by-step instructions to be followed before the start of each session. The user shall check that:
- –
The latest test results for the water and concentrates are within acceptable ranges.
- –
The machine has been completely disinfected.
- –
All the disinfectant used has been completely rinsed out; under no circumstances shall this step be left out.6,7
Ultrafiltration of the dialysate through an appropriate ultrafilter at low pressure is the method being used to obtain ultrapure dialysate. Most of these filters have synthetic membranes with a cut-off or molecular exclusion of about 40kD and high adsorptive capacity. Polysulfone and polyamide are the most commonly-used membranes in these filters. They are now being used to filter the dialysate just before the dialyser.8–10 They remove all kinds of particles, bacteria and endotoxins, many of them from the circuits of the haemodialysis machine. This prevents passing into the patient through the dialyser. Both online haemofiltration and haemodiafiltration require specific machines that include “cold sterilisation” of dialysate by two or more ultrafilters. To date, ultrafiltration of dialysate is the only method to have been proven effective in clinical practice. These ultrafilters have the following characteristics:
- 1.
Basically hollow-fibre filters composed of synthetic membranes (polysulfone, polyamide, posidyne)
- 2.
They have to be placed in series in the dialysate line
- 3.
They purify the dialysate by filtration (based on size exclusion and wall structure) and adsorption mechanisms (due to hydrophobic interactions)
- 4.
They have to produce a high-quality “ultrapure” dialysate
- 5.
They must guarantee microbiological quality equivalent to that required for parenteral solutions (infusion or haemofiltration solution)
- 6.
Their air tightness has to be monitored and they need to be disinfected periodically
- 7.
Sterilised ultrafilter with retention capacity of11):
- •
Bacteria: Log value>7 Pseudomonas diminuta
- •
Endotoxins: Log value>3–4 E. coli and P. aeruginosa
- •
With regard to centralised concentrate systems, we do not recommend bicarbonate concentrates in this guideline; and acid concentrates rarely become contaminated. We recommend that periodic descaling, washing and inspection shall be carried out.12
The ANSI/AAMI1 guidelines recommended that pure fluid for haemodialysis should have no more than 200 colony forming units (CFU)/ml and 2 endotoxin units (EU)/ml, and that for ultrapure fluid, there should be no more than 0.1CFU/ml and 0.03EU/ml. Meanwhile, the European Renal Best Practice (ERBP)2 published in 2002 recommended <100CFU/ml and <0.250EU/ml for pure fluid and <0.1CFU/ml and 0.03EU/ml for ultrapure fluid. The differences were unified by the International Organization for Standardization with ISO 11663 in 2009 and ISO 23500 in 2011,3 which recommended pure fluid with <100CFU/ml and <0.5EU/ml, and ultrapure with <0.1CFU/ml and <0.03EU/ml. According to all these guidelines, substitution fluid used for HD should have <10−6CFU/ml and <0.03EU/ml.
The Japanese Society of Nephrology4 establishes as microbiological standards in pure fluid <100CFU/ml and <0.05EU/ml measured at least once a month, and in all machines at least once a year. The standards for ultrapure fluid are <0.1CFU/ml and <0.001EU/ml. The level for alert should be 50% of the maximum allowable limit in any of the cases, except for endotoxins in ultrapure fluid. The Japanese society recommends <10−6CFU/ml and <0.001EU/ml for substitution fluid.
Ultrapure dialysate is absolutely necessary when used as substitution fluid for on-line haemofiltration or haemodiafiltration. To minimise inflammation in patients on haemodialysis, all dialysis units shall strive to achieve ultrapure dialysate for all types of haemodialysis. The routine use of ultrapure dialysate requires the fitting of specific ultrafilters in the dialysate circuit. (Level of Evidence B, 1)
The standards vary and some recommendations suggest reducing to 0.1CFU/ml, using sensitive microbiological methods, and <0.03EU/ml to define an ultrapure dialysate.5 The ultrapure fluid is achieved using three basic principles: ultrapure water; sterile ultrafilters interposed in the path of the fluid in well-designed HD machines; and strict compliance with disinfection procedures and microbiological monitoring.
According to all the recommendations, the current use of dialysers with high-flux membranes specially designed to allow convective transport is imposing the generalised use of ultrapure dialysate.6,7
The use of ultrapure dialysate has been accompanied by an improvement in the inflammatory state of the patient.8 The improvement of this state reduces the need for erythropoietic factors,9 reduces levels of B2 microglobulin10 and improves the lipid and myeloperoxidase profiles.11,12 Monocyte activation and apoptosis improve when using ultrapure dialysate.13 Its use also improves preservation of residual renal function14 and nutritional status.15 Ultrapure dialysate is associated with a decrease in vascular endotoxins, with the resulting improvement in vascular elasticity and systemic inflammation.16
A recent meta-analysis shows that the use of ultrapure dialysate in haemodialysis patients causes inflammatory and oxidative stress markers to fall, increases serum albumin and haemoglobin and reduces erythropoietin requirements.17 It also concludes that although those results are subrogate markers, it would be assumed that it must have cardiovascular benefits. Another study found increased survival in patients treated with high-flux dialysis when ultrapure dialysate is used, when compared with a conventional or pure fluid.18
Using ultrapure dialysate shall be mandatory in patients who are being dialysed with high-flux membranes, and in those with more convective transport such as online haemodiafiltration. The reason for this is the evidence that the use of ultrapure dialysate in these patients improves survival and cardiovascular events.19–21 In Spain, where 80–90% of patients are dialysed with high-flux membranes, it is mandatory that all machines have ultrafilters and that patients have the benefit of ultrapure dialysate.
In other areas unrelated to dialysis, ultrapure water is considered to be water whose conductivity does not exceed 0.5μScm−1, since it is very important that water cannot act as an electrical conductor, without specifying its intended use (photo labs, electronics manufacturing, etc.). By using the same term for haemodialysis water and setting the conductivity limit 5μScm−1, there are a number of considerations which have to be taken into account.
The conductivity limit required in dialysis is to ensure that the concentration of chemical contaminants in the dialysate, valued together, does not exceed levels considered toxic to patients. It is not therefore a problem of water acting as an electrical conductor. Total dissolved salts, solutes or solids (TDS), in mg/l, and conductivity are related as follows: the TDS are equivalent to the conductivity of water, in micro Siemens, multiplied by a factor ranging from 0.54 to 0.96 depending on the solute, although generally it is a value chosen somewhere between the two. According to this conversion, 2mg/l of a substance, or the sum of several substances dissolved in water, would have a conductivity of between 2.08 and 3.7μS. If instead of 2mg/l the TDS value was 4mg/l, although the concentrations of solutes are within the set range, the conductivity could be outside the range considered suitable for purified and ultrapure water for haemodialysis.
If we compare this with the maximum values of the chemical components that may be present in the water for haemodialysis, whether ultrapure or not, colliding see that certain elements can be in amounts up to 50mg/l (sodium) and therefore incompatible with the indicated conductivity. Thus, if we had water with microbiological, endotoxin and chemical levels well within the limits required for ultrapure water listed in the guide, but the conductivity was outside the ranges indicated, would it be correct to say that the water does not meet the recommended requirements for use in haemodialysis and ultrapure water?
Depending on the hardness of the feed water, pre-treated water can contain a high concentration of sodium chloride from the water softeners. This can be counteracted using reverse osmosis in series and an electro-deionization system, which would give us levels of conductivity below 0.5μS. This type of configuration is not usually used in haemodialysis water treatment systems, although it may be advisable in places with very hard feed water and/or where it is necessary to lower the conductivity.
It is also important to remember that gases dissolved in the water interfere with the measurement of conductivity, one of these being CO2, which can come naturally from the water itself or sometimes be added to obtain optimum pH. Other elements may also be added to correct the pH and affect the final conductivity.
Given the above data and arguments, it is acceptable that both purified and ultrapure water may have a higher conductivity than the recommended, provided the levels of chemical, microbiological and endotoxin elements are within the indicated range.
Nevertheless, it is important to stress that the conductivity achieved under optimal operating conditions, with all physical and chemical parameters being met, must be maintained over time, and that any changes, if they exceed a pre-set limit, have to be diagnosed and corrected if necessary. Monitoring conductivity allows on-the-spot control of the operation of the reverse osmosis equipment.
The Silt Density Index (SDI) shall be less than 5 in the feed water to the reverse osmosis equipment; some technical manuals for osmosis membranes or equipment recommend that it should be less than 3. When premature saturation of osmosis membranes occurs (loss of production flow rate and/or pressure variations), knowing the SDI and how it varies over time gives an indication of the suitability of the water quality. Measurement of SDI is regulated by ASTM International (formerly American Society for Testing and Materials).
This guideline requires a conductivity level and monitoring thereof because it is the method available in all water treatment systems and is the best method for warning us of malfunction.
The quality of the feed water to the haemodialysis unit is fundamental in terms of the design of the water treatment system and the results obtained. Feed water is synonymous with raw water, water to be treated and supply water. The quality of this water shall comply with Royal Decree (RD) 140/2003 on the quality criteria of water for human consumption and RD 865/2003, which establishes the health and hygiene criteria for the prevention and control of Legionnaires’ disease.
Apart from the obvious health and hygiene and quality criteria for potable water, both decrees set out actions for maintenance and upkeep in hydraulic systems.
This becomes of paramount importance in haemodialysis units located in hospitals, since these centres are generally considered “water managers” due to the volume of consumption and storage of potable water. Mismanagement or non-compliance with these RDs lead to a wide range of problems in the treatment of water for haemodialysis. The following are some common examples:
- •
Poor maintenance of the cisterns, which can lead to unwanted increases in all types of elements in the water which then cause serious defects in the haemodialysis water treatment system. The cisterns act as filters by decantation; excessive accumulation of silt deriving from decantation can under certain circumstances cause the silt to be drawn into the hydraulic distribution network, with the consequent problems.
- •
Poorly controlled chlorination, whether too much or too little, which can cause hazardous variations in water for haemodialysis.
This does not necessarily mean that it is the haemodialysis units that should be responsible for complying with the above RDs, but it shall be their responsibility to acquire documented verification that the actions and controls specified in the RDs have been carried out.
In hospitals, this job is generally the responsibility of the Preventive Medicine and/or Maintenance departments.
In external haemodialysis units, and even in small hospitals, there may be two contending factors:
- 1.
That due to the volume stored and/or consumed, they are considered as potable water “managers” and therefore have to comply with the RDs.
- 2.
That for the same reasons, they are not considered to be “managers”, so their role is limited to acquiring the documentation from the potable water supplier or local authorities, depending on which has ultimate responsibility for implementation of and compliance with the RDs.
When the supply of feed water to a haemodialysis water treatment plant does not directly come under a health authority but an intermediary, for example a neighbourhood association, tanker lorry, hospital storage tank, etc., guarantee of compliance with the above regulations shall be the responsibility of the people in charge of those bodies.
Premises where the equipment and ancillary facilities, such as air extraction and supply, drainage, electrical installations, etc. are located shall comply with the specific rules and monitoring for such components, with particularly close monitoring for elements whose influence may alter proper functioning or the results of the analyses performed (e.g. electrical protection, air vents above sampling points, etc.).
The decrees can be downloaded from:
https://www.boe.es/boe/dias/2003/02/21/pdfs/A07228-07245.pdf
http://www.boe.es/boe/dias/2003/07/18/pdfs/A28055-28069.pdf
Water treatment systems can be divided into 3 sections:
- –
Pre-treatment: feed or raw water tanks; chemical injection systems; filters; water softener; activated charcoal; microfilters.
- –
Treatment: One or more reverse osmosis systems and, optionally, an electro-deionising system.
- –
Post-treatment: UV lamps; ultrafilters; if applicable, treated water tank; and distribution system.
Shall be designed to guarantee a constant supply, whether via more than one supply connection, raw water tanks, pumps in duplicate, etc. The importance of this increases if the water treatment system is online, i.e. the product or treated water is delivered directly to the distribution network. If there are water tanks, they shall be opaque to prevent the growth of algae, and closed, with access for cleaning and disinfection. We recommend installing an individual meter to measure the consumption of the water treatment plant.
Chemical injection systemsCertain products, when added to water, can exercise different functions:
- –
Sodium bisulfite for removing chlorine/chloramines
- –
Acids for reducing the pH of the feed water and improving the efficacy of the activated charcoal
- –
Chlorine or ozone to control bacterial growth.
If any automated equipment for adding chemicals is installed, there shall be a device or equipment to monitor that parameter.
In principle, given the purpose for which we aim to obtain the particular quality of water, we should be thinking about the need to remove unwanted elements without having to introduce others that will have to be removed later.
Pressure regulatorResponsible for maintaining a constant pressure at the treatment system water inlet in order to prevent pressure surges that could lead to breakage of some element in the pre-treatment system. Pressure gauges shall be positioned at both the inlet and the outlet.
Pressure gaugesInstalled at various points along the treatment system, they allow us to visualise pressure losses at each point, helping us to identify potential failures or saturation of any of the elements by comparing pressures.
Pre-filtration – sediment filtersPre-filtration removes items in suspension1–7 that can cause premature clogging of the osmosis membranes or coating of the activated charcoal particles and softener resins. The system shall therefore be installed as the first element of pre-treatment and inserted at some point in the pre-treatment circuit.
It may consist of exclusion filters (wound or screen), calibrated sand bed filters, which can be regenerated by backwashing, or hydrocyclone filters. Even if the raw or feed water is confirmed as having minimal concentrations of particles in suspension, they should always be installed, as they cost little, including for maintenance, and the potential benefits are considerable.
In some cases, if there is a high concentration of particles in suspension, it may be necessary to install more than one item in series, gradually decreasing pore size.
The sand/anthracite filters have the advantage that they can be backwashed. The dimensions in terms of pore size or discrimination capacity of the sand in the filters can go as far as the exclusion of particles >5μm, but typically about 20μm. To remove even smaller particles, the larger pore-size filters will need to be followed by additional microfilters in series. The choice of filters shall depend on the characteristics of the feed water, always placing them in order from larger to smaller pore size. The filter container shall be opaque to prevent growth of algae. The filter elements must have pressure gauges at the inlet and outlet to detect pressure drop as an indicator of fouling.
In the hydrocyclone filters, the filtering process takes place through the discs with the water to be filtered entering via a tangential inlet causing a spiral flow, the hydrocyclone effect, which keeps dirt particles in suspension away from the filter element and drags them helically to the bottom of the filter housing. The filter element is a slotted disk cartridge made of polypropylene that ensures high resistance and quality of filtration; providing the filter with a long life. Filtration grades from 5 to 200 microns. Cleaning involves manually cleaning the filter cartridge; easy disassembly of the filter cartridge allows it to be cleaned more thoroughly. Cleaning can be automated by incorporating an automatic purge kit.
Water softenersTheir job is to remove calcium and magnesium (hardness of the water) by ion exchange when passed through a resin bed.1–7 Hard water can cause precipitation of calcium carbonate.
First of all, the precipitation can damage elements such as the osmosis membranes. Secondly, if the hard water reaches the patients through the distribution network, it can cause so-called hard water syndrome.
The water softener contains ion exchange resins which exchange sodium ions (Na+) for calcium (Ca++), magnesium (Mg++) and other polyvalent cations that make the water hard. Once the resin is saturated with Ca and Mg, it is regenerated with brine. This causes the resins to newly acquire cations of sodium Na+ and displace the Ca++ and Mg++ from the resin.
Water softeners are usually installed in a twin configuration, i.e. two resin filters controlled by one or two heads. Twin water softener (Duplex) or two independent parallel water softeners. The control valve will be automatic and it shall have a salt storage tank.
The operation of the filters may be:
- –
Alternative: One of the filters is operating and the other regenerating or in waiting phase.
- –
Parallel: The two filters work in unison, but they never regenerate simultaneously.
This is due to the fact that the regeneration process is slow because, once saturated with Ca and Mg, the resins need contact time with the brine for cation exchange. In addition, a backwash is necessary for expansion and cleaning of the resin.
Another important aspect to consider is that the time between regenerations is sufficient; to guarantee that the contact time between the water and the salt (in the salt tank) is sufficient to generate the brine.
The regeneration system should have a safety mechanism that prevents the brine from the regeneration from passing into the treated water.
The regeneration shall be automatic and may be programmed according to:
- –
Volume of circulating water: This volume shall be programmed according to the number of litres of resin, the resin's exchange capacity and the hardness of the water.
- –
Time: Performing regeneration overnight. Hardness tests should be carried out on the softened water prior to regeneration. Filters must do a regeneration at least once a day.
If the water is extremely hard, more than one battery of water softeners may be required. In such cases, it is important to bear in mind that water softeners can produce large amounts of sodium as a result of the cation exchange in the resins.
The salt used for regeneration shall meet the specifications set out in RD 1424/83 and CE 91155 EWG, as using unrefined salt or sea salt directly can result in undesirable elements in the subsequent steps of the treatment system, such as particles, iodine, etc.
Charcoal filterThe job of the charcoal filter is essentially to retain chlorine and/or chloramines. Chlorine can cause serious damage to some osmosis membranes and if it reaches the patient, can cause haemolysis.
The activated charcoal filter removes chlorine and chloramines added to the water to prevent bacterial contamination by adsorption; it can also remove organic substances dissolved in the water.1–10 The charcoal filter shall be installed post water softener to avoid leaving the earlier steps of the treatment unprotected against microbial contamination after removal of chlorine and chloramines.
The size shall be determined according to the level of chlorine in the water. They shall contain a suitable charcoal both in terms of source and activation. We recommend using 12×40 acid-washed granular activated charcoal (GAC) with a minimum iodine number of ≥900.
Whenever feasible, replaceable charcoal cartridges shall be dispensed with in favour of backwashing charcoal filters. This is because the charcoal cannot be regenerated, so gradually becomes exhausted. This means that with replaceable cartridges, chlorine or chloramines may be present due to partial exhaustion and not be detected immediately.
Backwashing means circulating the water in the opposite direction inside the charcoal filter which makes it swell or expand. This is important since while in operation, the charcoal becomes compacted. Channels can form, allowing the water to flow through and thereby minimising contact with the charcoal, leading to the risk that the chlorine and/or chloramines may not be removed. This process helps in part to preserve the charcoal from possible contamination by introducing water through part of the internal circuit in which it always circulates without the presence of chlorine. The backwash shall be performed at least once a day. It is generally programmed to run overnight when there is no demand for water from the unit.
The design and size of the filters shall be suitable to obtain a total EBCT of over 7minutes, although we recommend over 10minutes.
where V=volume of charcoal in litres; Q=estimated maximum flow rate through filter (l/min); recommended EBCT at least 10min; Example: For an RO with a maximum (feed) of 40l/min, 400l of charcoal should be used (400/40=10min).As it cannot be regenerated, the charcoal shall be changed regularly to prevent it from releasing adsorbed substances due to saturation, micro particles of charcoal that have been reduced by friction, etc. More than one filter may be used and they may be installed as follows:
- A.
In series2,8,10: one filter after another. This guarantees that the water travels at the highest speed possible and if one of the elements fails, the other continues working. The disadvantage of this is that, if the first filter removes all the chlorine, the second filter will never have contact with chlorine and chloramines, not even during backwashing, as the first filter will remove all the chlorine under normal operating conditions, and this could lead to bacterial contamination. It is essential that the level of chlorine/chloramines can be measured independently in both filters.
- B.
In parallel: the water enters both filters simultaneously and therefore only passes through one filter. In this case, the water circulates more slowly which increases the contact time (EBCT). If one of the elements fails, it means that some of the water will still contain chlorine and/or chloramines. It has the advantage that the two filters do the backwash with chlorinated water and that if one fails, the line can be cut off while the second line remains operational.
After the filter, there shall always be a microfilter, of about 5μm, or even better 1μm, to guarantee that in the event of release of charcoal particles, they cannot pass through to subsequent parts of the treatment system (see manufacturer's requirements for the water treatment equipment in the subsequent step).
A.2.1.2TreatmentReverse osmosisBased on the physical principle of osmosis across a semipermeable membrane, the flow of water is reversed by pressure from a hydraulic pump.1–7,11–16 The membranes are capable of retaining 90–99% of ions and 95–99% of organic elements. The yield is determined by the product and reject streams. The product or permeate flow is the water that crosses the osmosis membrane and is sent for use. The reject or concentrate flow is the water that does not cross the membrane as it contains high concentrations of elements dissolved in the water that cannot pass through, and it is sent to drain or partially or entirely fed back to the system; for one-stage osmosis systems, this generally tends to be 50–50, although it can vary depending on the design of the equipment, the characteristics of the raw water, the pretreatment and the quality to be obtained with the above parameters.
The effectiveness of the membrane or ion rejection is determined by the input and output conductivity (electrical parameter; inverse of resistivity), i.e. the conductivity of the water when it goes into the osmosis system and comes out of it (permeate) ready for use or to continue on to subsequent processing elements. The formula usually applied to determine the efficacy or ion rejection is:
Obviously, the more effective, the higher the water quality, but this can be misleading because a very high input conductivity level will be reflected by the permeate or outlet also having high conductivity, even with efficiency above 99%; conversely, a low input conductivity will be reflected with low outlet or permeate conductivity, but with low efficiency (<90%). Conductivity should be used as the monitoring parameter for proper functioning of the system; when we compare the results of the chemical analyses with the usual value, it will tell us that there are no variations in the ionic components of the water. There are parameters that can affect the conductivity reading but without implying any reduction in the water quality, such as the presence of microbubbles.
In addition to conductivity, the pressure the membranes are subjected to and the permeate and reject flows serve as indicators of the water quality once established according to the manufacturer's specifications. The number of membranes used will be determined by consumption of treated water; obviously this has to be as finely adjusted as possible, since using the bare minimum number of membranes can mean having to raise the pressure over time (saturation of the membranes) and even having to increase the permeate flow with respect to the concentrate (reject) flow, which would lead to a decrease in the final quality.
Osmosis equipment has to be periodically descaled and disinfected; this work will essentially depend on the quality of water entering the system, but it should be avoided as far as possible, as both operations result in a decrease in the effectiveness of the membrane.
The correct design and subsequent monitoring of the pre-treatment components (pre-filtration, water softening and dechlorination) are essential for the proper operation of the osmosis system, as failure of a component or poor design can have a significant impact on the osmosis membranes. For example, the following aspects are crucial: complete removal of chlorine to prevent perforation of the membrane; hardness removal to prevent premature clogging of the osmosis system; prevention of excessive presence of matter in suspension, including elements from the pre-treatment (charcoal), as this can cause contamination, clogging, etc. Another factor that can influence the membranes is the water temperature; at higher temperatures, the membrane increases water production, but this may result in a lowering of quality; at lower temperatures, the opposite occurs. The osmosis system shall be equipped with audible and/or visual alarm systems which signal when conductivity deviates from the allowable limits.
Osmosis membranesThe quality of pre-treated water must be taken into account when designing the reverse osmosis system, as the operating parameters will have to be set accordingly. Consult the manufacturer's recommendations on the design and operation of a reverse osmosis system for the membranes to use.
The figure below shows a basic idea of the difference between one-stage (top) and two-stage (bottom) osmosis:
- •
One-stage: classic configuration, may have a back feed from the outlet to the inlet in order to improve yield (redundant flow). Water consumption is high - good yield would be 40% reject, which is sent to drain, and 60% product; the usual is 50%. If the incoming water has high levels of dissolved elements, a certain proportion of them will pass through the membrane, as it retains a percentage (90–99%). In the event the osmosis fails, dialysing is not recommended.
- •
Two-stage1,6,11–13: The reject water from the second stage is recovered in full and sent back to the inlet. Lower water consumption, since the configuration of the system means it can work with up to 20% reject. Even if incoming water inlet has high levels of dissolved elements, very good quality water is achieved, as the second stage acts only on the permeate water from the first. This configuration is recommended not only due to the better water quality obtained, but also because, if either of the stages fails, work can continue with the other, producing water quality similar to a one-stage system. Each stage shall have its own pump system.
The equipment shall have CE marking and should be certified as medical device (medical electrical equipment=medical device).
Deionisers1–6,11,12,14–16Usually added as an alternative to a second stage of osmosis.
Between 1% and 10% of the ions are not retained by osmosis (one-stage). Therefore, if there are very high concentrations of ions pre-osmosis, there may also be a high presence in the output. We recommend use of deionisers in places with high ionic load. There are currently two classes of deionisers:
- •
Electro-deionization system1,11: mixed-bed resins with small volume separated by membranes and subjected to a polarised electric field, which causes anions and cations to migrate to the corresponding electric pole through the membrane. A stream of waste water between the other side of the membrane and the corresponding electric pole causes flushing of the ions that have crossed the membrane, i.e. continuous regeneration. If a deioniser is necessary, an electro-deionization system shall be used.
- •
Ion exchanger1–5: Works in a similar way to the water softener, but in this case it is a mixed bed: an anion and a cation exchanger. They may be in one tank or two different tanks. Exchanges Na+, K+, Mn+, etc. cations for hydrogen ions (H+) for the cation exchange bed and HCO3, Cl, F, SO4, etc. anions for hydroxide (OH−), and as a result of the two processes, H2O is produced. Ion exchangers can easily become contaminated due to their large volume and the lack of bactericidal elements in the water; if they become saturated, they start releasing retained ions, so have to be constantly monitored by a conductivity meter at the outlet, must have an alarm system and require regular epidemiological testing. Due to the aggressive agents that have to be used for regeneration (acid for the cation column and caustic soda for the anion column), this is usually done outside the centre. For that reason, the use of ion exchangers is not recommended, particularly in view of the alternative of using an electro-deionization system.
Generally used in addition to one-stage reverse osmosis, or both simultaneously, when storing treated water to prevent contamination in the tanks from passing into the distribution network. Submicron or ultrafilters mainly retain bacteria and other elements dissolved in the water; depending on the pore size, they will also retain endotoxins. The endotoxin adsorption capacity varies according to the type of membrane. Some of these filters are very similar to a large dialyser, having very similar membranes, with some of the water being directly rejected. Breakage of these filters due to overpressure or excessive prolongation of their useful life could allow contaminants through to the rest of the circuit. The pore size selected will depend on the previous system. If ultrafilters are placed pre osmosis, they can quickly become clogged. If the osmosis provides water free of high concentrations of dissolved elements (bacteria, ions, etc.), the filter to use would be one which acts as a barrier to endotoxins, with a size of <1 angstrom approximately.
The integrity and saturation of the membrane shall be guaranteed by installing gauges at the input and output to measure changes in pressure.
To produce ultrapure dialysate, ultrafilters are necessary in the dialysis machines. For “online” haemodiafiltration, a second ultrafilter is necessary to obtain substitution fluid.
Ultraviolet lampShort-wave ultraviolet light is bactericidal1–3,5,17 and this can result in the massive presence of endotoxins; UV lamps shall therefore always have a system, either ultrafilter or reverse osmosis, capable of removing them. They should be used for treated water tanks susceptible to contamination, etc.
They must be very well designed according to the flow and speed of the circulating water. Other elements in suspension in the water will greatly reduce the lamp's effectiveness.
Maintenance: Lamps have to be replaced once a year to ensure maximum efficiency. Lately, use of this type of system is falling due to the constant monitoring and maintenance required, in comparison with dual RO, which allows more controlled water purification and no interruption of the dialysis due to failure of one of the RO units.
Storage tanksWe recommend that storage of treated water shall be avoided as far as possible, especially post osmosis treatment, because of the risk of contamination, the difficulty of disinfecting it and the few barriers from this point to the patient. Should a storage tank be necessary, it shall be opaque to prevent growth of algae and have a 0.45μm hydrophobic vent filter. Tanks shall also be equipped with a UV light system and an ultrafilter for the retention of endotoxins at the outlet, and have a conical bottom so that they drain from their lowest point. Flexible and pressurised tanks shall be avoided. It shall be possible to carry out full disinfection.
Distribution systemsThe distribution system guarantees the supply of water to dialysis machines and local acid-concentrate production systems. We recommend that it shall be colour coded to indicate the direction of water flow. The distribution system must be designed to maintain the chemical and microbiological quality of water and shall therefore meet the following criteria:
- –
Continuous loop with as short a journey as possible
- –
Minimum number of connections
- –
Minimum pressure drop
- –
Materials compatible with the conditions of use (e.g. supply, disinfection, cleaning)
- –
Not release chemical substances or nutrients for microorganisms (copper, aluminium, lead, zinc, etc.)
- –
Material with low surface roughness
- –
Opaque
- –
At least one sampling point at the end of the distribution loop
- –
Avoid dead zones and minimise the distance between the loop and the connection points to the machines.
The choice of materials shall depend on the proposed disinfection system. The following table is a guide to material compatibility with disinfection products.
Material compatibility with disinfection products.
| Material | Sodium hypochlorite (Bleach) | Peracetic acid | Formaldehyde | Hot water | Ozonea |
|---|---|---|---|---|---|
| PVC | × | × | × | × | |
| CPVC | × | × | × | × | |
| PVDF | × | × | × | × | × |
| PEX | × | × | × | × | × |
| SS | × | × | × | × | |
| PP | × | × | × | × | |
| PE | × | × | × | × | |
| ABS | × | ||||
| PTFE | × | × | × | × | × |
| Glass | × | × | × | × | × |
PVC, polyvinyl chloride; CPVC, chlorinated polyvinyl chloride; PVDF, polyvinylidene fluoride; PEX, cross-linked polyethylene; SS, stainless steel; PP, polypropylene; PE, polyethylene; ABS, acrylonitrile butadiene styrene; PTFE, polytetrafluoroethylene.
× indicates compatibility.
The user shall verify the compatibility of the germicide with the distribution system materials. When considering compatibility, joints, junctions and the tubing material shall also be included. Consideration shall also be given to germicide concentration and the duration, frequency and conditions of exposure to be applied (flow, pressure, temperature).If the length of the junction from the loop to the machine connector is more than three times the diameter of the loop, common in hose-type connections, this section shall be included in routine disinfection.We recommend that the distribution system shall be certified as a medical device.
Distribution system disinfection systemsDisinfection can be carried out by various methods:
- (a)
Chemical disinfection: performed according to the schedule defined after plant validation. For chemical disinfection, we recommend using sodium hypochlorite or peracetic acid. Avoid the use of sodium hypochlorite for disinfection of osmosis membranes as it can damage them.
Most reverse osmosis systems these days enable easy disinfection of the equipment itself and the distribution system (if connected online).
Following disinfection, the absence of traces of disinfectant shall be verified before performing dialysis.
- (b)
Thermal disinfection: Enables disinfection to be performed automatically. Shall have a system for temperature monitoring at the most distal point to ensure that the temperature required by the manufacturer during the disinfection cycles is reached. Important that the circuits recommended for heat sterilisation are those manufactured in cross-linked polyethylene (PEXA), acrylonitrile butadiene styrene (ABS plastic), PVDF (polyvinylidene fluoride), PTFE (Teflon) or pharmaceutical grade stainless steel.
PVDF
http://www.resinex.es/tipos-de-polimeros/pvdf.html
http://www.gfps.com/content/gfps/country_US/en_US/products/piping/pvdf-sys/sygefstd.html
http://fgf.hu/storage/file/Product%20Range%20International%202011%20-%20SYGEF%20PVDF_GFDO_8260_4b.pdf
PTFE
http://www.fluorotherm.com/technical-information/materials-overview/ptfe-properties/
http://www.merefsa.com/productos/ptfe-politetrafluoretileno/tubos-lisos-y-coarrugados-de-ptfe_pid23.html
http://www.inalcoa.net/catalogo/plasticos-2/ptfe-marca-teflon-%C2%AE/
- (c)
Ozone disinfection: Enables disinfection to be performed automatically. Where an ozone disinfection system is used, the concentration of ozone in the air shall be monitored to ensure that permitted limits are not exceeded.
The water treatment system shall have pressure gauges, flow meters, sampling points in the right places for monitoring the water purification process, and valves that enable certain individual elements to be bypassed to facilitate repair or allow operation of the system in case of failure.
Electrical circuits shall be separated from the hydraulic circuits and adequately protected from possible hydraulic leaks.
Characteristics of the water treatment facilityThe haemodialysis water treatment facility should be located as close as possible to the haemodialysis unit (less than 25 metres). The surface area will be consistent with the number and size of the components. The design of the water treatment facility has to take into account the weight of all the equipment (full of water) and guarantee that the floor will support it.
We recommend that it shall be at least 25m2. The floor and part of the walls shall be waterproofed. The room must have a drain to evacuate more than 5000 l/h. It shall also have a central sump, which shall be the lowest point of the room. The room shall be well ventilated with temperature maintained between 15 and 30°C. There shall be easy access for supplies and, if possible, it shall have its own access separate from that to the haemodialysis unit.
If it is has a thermal disinfection system, the drainage system shall be able to withstand high temperatures (>90°C).
Location of the water treatment facilityIt is very important that the facility is close to the haemodialysis unit and it is not at all advisable to have one water treatment facility for use by two units some distance apart. Long runs are not acceptable due to the risk of contamination.
A.2.2Components of the concentrates for dialysisThere are two types of concentrate:
- –
Acid: with ions (Na, K, Ca, Mg, Cl), with slight variations (mainly in K and Ca) according to the medical prescription, acetic acid to lower the pH and prevent the precipitation of calcium carbonate that forms when Ca in the acid concentrate comes into contact with bicarbonate when making the dialysis fluid, and sometimes glucose.
- –
Bicarbonate: sodium bicarbonate as buffer, sometimes with sodium chloride.
They can be purchased in containers or small bags to supply directly to the dialysis machines, in which case no production system or distribution network is required and manufacturing shall be the responsibility of the manufacturer.
They can also be supplied in large tanks, in which case distribution networks shall be required, but manufacturing shall still be the responsibility of the manufacturer.
If concentrates are to be prepared, there shall be a system for mixing purified water and salts, with storage and distribution for them. It shall also be mandatory to have the appropriate permit and signature of the person responsible for preparing that product.
A.2.2.1Concentrate preparationWe do not recommend preparation and storage of dialysis concentrates on-site due to the inherent difficulties and the lack of current regulations in this regard, particularly in the case of bicarbonate concentrate as it is easily contaminated. The components of a concentrate preparation system shall be made of compatible materials to prevent chemical or physical reactions affecting the concentrate purity. Purified water shall be available and appropriate checks shall be made to ensure quality standards and chemical and bacteriological purity. We do not recommend preparing, storing or distributing bicarbonate concentrates in network due to its instability (it is rapidly degraded to give CO2 and H2O) and frequent contamination (especially at room temperature). For bicarbonate, we recommend the use of solid micronised salt in cartridges; this salt is dissolved in warm water in the machine during the dialysis session for immediate use, ensures better dissolution, stability and purity and is currently the best system for preparation of the bicarbonate dialysis bath.
A.2.2.2Concentrate distribution- (a)
Compatible materials
All components used for the distribution of acid concentrates for dialysis (storage tanks, pumps and distribution network) shall be made of materials compatible with the fluids (plastic materials or stainless steel) so that they do not interact and do not produce chemical contamination.
- (b)
Design
It may be distributed by gravity from an overhead tank or via a pressurised circuit by a pump.
In the case of distribution by gravity, the tank shall be a cone bottom tank with outlet at the bottom and a spray mechanism to enable cleaning and disinfection. It shall be closed and have a 0.2mm hydrophobic air intake filter to prevent contamination, and have a level alarm system.
- (c)
Acid concentrate distribution
Both the distribution system and the connections to the dialysis machines shall be colour-coded in red.
There are two distribution layouts: tree system; and loop system. The tree system delivers the concentrate to the points of use along branches. The loop system delivers the concentrate to the points of use by way of a loop and continuous flow.
The distribution networks for the different acid concentrates and the connection points to the dialysis machines shall be colour-coded in red with initials for identification.
Although the acid concentrates do not easily become contaminated with bacteria, the circuits shall be closed to prevent evaporation or non-bacterial contamination. Regular disinfection is not usually required but periodic descaling, washes and inspections are necessary.
- (d)
Bicarbonate concentrate distribution
Both the distribution system and the connections to the dialysis machines shall be colour-coded in blue and shall be different from those in the acid network to prevent connection errors.
Given that the bicarbonate concentrate is an excellent medium for bacterial growth, the system shall be designed to enable frequent periodic disinfection with acid products as descalers, active oxygen as cleaner and active chlorine or aldehydes as disinfectants. The system may also be equipped with an ultraviolet radiation system or an ozone generator.
Definition: A haemodialysis machine is a component designed to mix the concentrated electrolyte solutions or powder cartridges with the treated water to an electrolyte concentration, pH and temperature determined by medical prescription, while at the same time being properly degassed.1,2 The quantity of electrolytes diluted in the water is controlled by monitoring the conductivity; the pH of the final solution is sometimes also determined. The temperature is regulated to be suitable at the point of contact between the bath and the patient. The absence of air in the form of microbubbles shall also be guaranteed.
The drainage system for the machines shall be in “free fall”; this means that there shall be no contact between the drain from the machine and the plumbing system for the building in order to prevent contamination from the sewage system from accessing the machine.
- •
Compliance of haemodialysis machines with the standards UNE-EN 60601-1-6, 2010/UNE-EN 60601-2-16:1999/CORRIGENDUM16 and subsequent if any; among other aspects, these standards cover monitoring the preparation and temperature of the dialysis bath. Dialysis machines shall also comply with other standards for electrical and medical components by which they may be affected and shall have CE marking.
- •
Compliance with the manufacturer's recommendations for preventive maintenance work1,2and particularly monitoring of preparation of the haemodialysis fluid (degasification, heating and electrolyte composition monitored via the conductivity): proper operation shall be monitored and verified according to the manufacturer's specifications. All maintenance and repairs shall be carried out by qualified staff, either the manufacturer or distributor of the machine or the properly trained technical staff of the haemodialysis unit with the precise technical information, this being the responsibility of the manufacturer or distributor of the machine. Measuring devices external to the machine used for monitoring and verification of the proper preparation of the haemodialysis bath should be checked periodically, either by specialised laboratories, the product manufacturer or using standards specifically for that purpose.
- •
Disinfection and descaling after every dialysis session, with absence of any traces of disinfectant agents in the hydraulic circuit before the start of the new session.1,12,13,17 Minimum guarantee of proper operation of the machine by running autotests on the main parameters. Proper descaling has become an essential part of guaranteeing, on the one hand, disinfection of the machine after a dialysis session to avoid possible contamination to the next patient and, on the other, the proper operation of the machine to avoid continuous depositing of material in its various components (biofilm) that cause errors in the different bath preparation parameters.
With the use of certain disinfectants or other circumstances, checks shall be implemented after each disinfection, to ensure the absence of any traces of disinfectant agents before commencing a new dialysis session, using test strips, colorimetry, etc.
The completion of the machine autotests before the start of each dialysis session has become an important element in guaranteeing the proper operation of the machine and thereby significantly increasing patient safety, while providing a guarantee of the machine's reliability for the medical, nursing and technical staff. The autotests generally consist of the simulation of different conductivity and temperature values to be compared with default values in the machine itself and which shall not exceed the default deviation value. The machines run another series of tests, not discussed here, where another set of control parameters guaranteeing its proper functioning (blood leak detector [BLD], UF, hydraulic circuit seal, flows, electronic controls and power supplies, etc.) are checked. If any of the tests are failed, it may be possible to start the dialysis session under the responsibility of the operator. We do not recommend doing this, and particularly not repeating session after session. Therefore, if a machine fails any of the tests, the test shall be repeated and, if it still does not pass, the machine shall be withdrawn as soon as possible; in the event of having to perform haemodialysis with a failed test, the operator shall closely monitor the operation of the machine, following the manufacturer's instructions.
- •
Inclusion of ultrafilter on the dialysate line1,3,11–13. To complement the use of high-quality water, the use of ultrafilters in machines that filter the dialysate is strongly recommended, regardless of the dialysis technique used (standard/online) or the type of dialyser (high or low flux).
Their use is mandatory when the “online” dialysis technique is used or when the dialysers are high-flux, in order to obtain ultrapure dialysate. The use of this technique and high-flux dialysers means more contact between the patient's blood and the dialysis bath, so not only is it vital to prevent the possible presence of pyrogens from the water but also from the dialysis concentrates, especially containerd bicarbonate.
The inclusion of an ultrafilter in the bath line should include as intrinsic to installation the possibility of their descaling and disinfection as simply another part of the circuit, unless they are single-use, in which case it shall be strictly necessary to comply with the above requirement. All the equipment necessary for producing the dialysate must meet certain requirements and technical specifications in order to achieve and maintain a high quality.18–27
- •
Recommendation on the useful life of the machines. Dialysate is produced in the dialysis machine; the age of the machine, regardless of how well-maintained, is related to the safety and efficiency of that process. We recommend that haemodialysis machines shall have a useful life not exceeding 7 years or 30,000hours of operation.27,28Recommendation on reserve machines. Haemodialysis units shall have reserve machines available for use. The number, between 12% and 20% of the stations in operation, may vary depending on the age of the machines, availability of technical services and care in isolation or special units. There shall be protocols in place for their maintenance.
Metabolically, bacteria can be classified into three large groups:
- –
Photosynthetic bacteria capable of producing carbohydrates using solar energy (cyanobacteria).
- –
Chemosynthetic bacteria capable of synthesising their nutrients and obtaining energy from inorganic compounds.
- –
Heterotrophic bacteria, which need organic compounds for their development. This is a broad and diverse group that includes symbiotic, saprophytic and pathogenic species. The term heterotrophic is commonly used as a generic name for water bacteria with few nutritional requirements.
The majority of the bacteria detected in dialysis water are gram-negative bacilli, generally glucose non-fermenting bacilli. Using identification systems designed for clinically relevant bacteria, the genera most commonly found are Pseudomonas, Stenotrophomonas, Burkholderia, Achromobacter, Acinetobacter, Ralstonia, Agrobacter, Moraxella etc. Less common are gram-positive bacilli (identified as Corynebacterium) and enterococcus. Fungi are not uncommon in dialysis water but they are found in lower quantities than bacteria. The most commonly isolated fungi are Candida parapsilosis and dematiaceous filamentous fungi. There are notable differences in the microbial flora between one hospital and another.1–4
The number of viable bacteria, capable of reproduction, in the water is determined by cultivating a known quantity of water in a solid culture medium (agar plate) and counting the number of visible colonies. The number of colonies is expressed as colony forming units (CFU) and it depends on the volume of fluid inoculated, the composition of the culture medium, temperature and incubation time. For historical reasons, the medium containing sodium chloride, pancreatic enzyme digest of casein and papaya enzyme digest of soybean meal (commonly called TSA) has become the reference medium for bacterial quantification studies in dialysis water. Media poor in nutrients, such as R2A medium,5 incubated at room temperature for 14 days, detect more CFU than richer media incubated for less time or at a higher temperature.6 R2A medium is superior to TSA, even if the other culture conditions are the same.7–10 This creates a conflict: The methods formerly recommended as reference by the Spanish Royal Pharmacopoeia, the European Pharmacopoeia and the AAMI underestimate the number of bacteria present in water. Moreover, with the application of more sensitive methods, it becomes more difficult to maintain the set maximum level of CFU. Numerous studies have found no correlation between the number of CFU in water and the amount of endotoxin.
To facilitate comparison of our results with those of other centres, we recommend that commercially available culture media should be used routinely and that they be supported by broader experience. To this end, we recommend the use of Reasoner 2A plates.10
The count of algae and fungi in dialysis water and its clinical significance is a little studied phenomenon. Arbitrarily, the maximum allowable fungal count is set at a number 10 times lower than bacteria.
We do not recommend any identification of microorganisms recovered in culture as it has not been demonstrated that the clinical significance of the counts varies according to the species present.
Higher bacterial counts are often detected in dialysate than in the dialysis water (see Guideline 3-1).8 The bicarbonate concentrate is a medium particularly easily colonised by bacteria. Units with distribution circuits for bicarbonate concentrate shall strictly monitor these circuits. Bacteriological monitoring of dialysis concentrates is particularly difficult to standardise since the microorganisms that reproduce in this environment have developed coping mechanisms that hinder their detection in culture. The sensitivity of detection of these bacteria can also be improved by making media poor in nutrients to which different concentrations of bicarbonate9 or sodium chloride11 are added.
The Spanish Royal Pharmacopoeia does not set specific limits for fungal contamination in the dialysis water. What it does do is set out that a specific culture medium shall be used for fungi, Sabouraud or similar, and that the incubation temperature shall be 20–25°C.
A.3.1Methods for collecting samples and culturesTaking samplesSampling methodMicrobiological monitoring of purified and highly purified water shall be done weekly during the month-long validation phase. Subsequently, and during the maintenance phase, monitoring shall be carried out at least once a month.
Sampling points: In the validation period, samples shall be taken of the following: feed water; softened water; the treated water immediately pre and post RO; the distribution loop and from at least 10% of the water intakes for the machines; and the dialysate on entry into the dialyser. In the maintenance period, it is not necessary to take samples in the pretreatment, unless significant contamination is detected of the treated water.
The sampling of the water intake ports on the machines should be done at the start of the dialysis session.
Taking samplesThe sampling point shall not be cleaned with disinfectants such as hypochlorite, acetic acid, peracetic acid, etc. Use of 70% alcohol is acceptable as long as it is then left to fully evaporate. We recommend the use of sterile gloves and that the task of sample collection is performed between two people, with the aim of minimising cross-contamination.
If instruments are used to open the safety valve and allow water to flow out of the dialysis machine connection ports, these elements shall have been previously sterilised (autoclave or gas).
The bacterial load of each dialysis water sampling point shall be taken after letting the stream run over a strictly-controlled period of time, i.e. 1minute or, preferably, until a fixed amount of one litre of water has drained, since the first decilitres of water usually have a significantly higher bacterial load.
The dialysate shall be taken from the machine using a syringe or a sterile container.12
The samples can be collected in any sterile glass or plastic container. A 50ml urine culture container is suitable for purified water. The containers should be labelled beforehand, stating the collection site. To determine bacterial load in highly purified water or dialysate, a sample of over 100ml is required.
The containers containing the samples should be kept on ice or refrigerated at 4°C (between 3 and 6°C) until processing for culture. The culture should be done in the shortest time possible, within a maximum of 24hours.
Culture procedureThe number of colonies that can be reliably counted with the naked eye is between 50 and 200. The methods proposed below are adapted from the rules of the Spanish Royal Pharmacopoeia, adjusting the volume inoculated to obtain the highest accuracy in the range closest to the cut-off. Counts above 200 or below 50 may only be taken into account assuming that they do not correspond to an approximation of the actual number, except if there are other plates on which other amounts or dilutions of the sample have been spread that enable greater accuracy in the count. It may sometimes be necessary to change the volume of liquid spread or dilute samples in sterile water to accurately quantify those with a very high level of contamination. The inoculated volume should never be less than 0.1ml.
To read the number of colonies, a magnifying glass (4–10×) should be used, clicking with a hand tally counter or a punch while counting the colonies. If the mass dilution method is used, they can be marked with a marker pen on the underside of the plate.
The culture media most cited are TSA, TGEA and R2A. In this guideline we recommend R2A, as it is superior in its ability to detect microorganisms in water.10 Blood agar is not recommended, although a comparative study found that it performed similarly to TSA.
Plate count methodThe colony count can be done in three ways: by surface-spread of the sample; by incorporating into a liquid agar medium (serial dilution or dilution in agar); and filtration through membrane. The Spanish Royal Pharmacopoeia, in reference to microbiological monitoring of products not necessarily sterile, recommends spreading each sample and each sample dilution in duplicate and using selective media (Sabouraud) for cultivation of fungi. Given the wide assay ranges for counts in dialysis water and the multiple factors involved, it may not be essential to quantify the number of viable bacteria so accurately.
Surface-spread methodPurified water: Use petridishes with R2A. Use aseptic technique to inoculate each plate with a volume of 1ml of the sample and spread with an angled sterile loop over the entire surface of the medium. After the agar has completely absorbed the inoculated fluid, turn plates upside down for incubation. The usual plates (9cm diameter) may take 1 hour to absorb 1ml of water. This time can be reduced by using plates of 14cm in diameter.
Incubate the plates at 23–27°C for 7 days, unless a shorter period allows a more reliable count to be obtained (large colonies that can hide other smaller colonies). Record the number of colony forming units per millilitre.
Membrane filtrationUse membrane filters with a nominal pore diameter of 0.45 microns or less, and whose effectiveness in retaining bacteria has been demonstrated. For example, cellulose nitrate membranes with a nominal pore diameter of 0.22 microns. To force the liquid through the membrane, vacuum devices connected to the drain terminal of the filter holder can be used. Filtration equipment is designed to allow easy transfer of the filter to the culture medium.
If the aim is to detect microorganism concentrations above 0.1CFU/ml (highly purified water), between 100 and 1000ml should be filtered. The Spanish Royal Pharmacopoeia recommends washing each filter three times by passing through 100ml of a suitable liquid, such as a solution of peptone-buffered sodium chloride at pH 7.0, every time. If validated, fewer than three washes may be done. Transfer one of the membrane filters, intended primarily for bacteria count, to the surface of a plate with suitable solid medium such as the R2A solid medium, and one filter, intended for fungal count, to the surface of a plate with suitable solid medium such as Sabouraud. Incubate the R2A and Sabouraud plates at room temperature (20–25°C) for 7 days, unless a shorter period allows a more reliable count to be obtained. Select the plates with the greatest number of colonies but fewer than 100 colonies and calculate the number of colony forming units per millilitre of sample.
Currently, most of the guidelines, including ISO 2014, recommend taking readings of cultures at 7 days after spreading and maintaining the plates at room temperature. This methodology has shown greater sensitivity than those used previously.12–14
Annex to appendix 3Composition of culture mediaReasoner R2A medium (modified according to the Royal Spanish Pharmacopoeia formula, referred to as medium S).
| Composition | |
| Yeast extract | 0.5g |
| Proteose-Peptone | 0.5g |
| Hydrolysed casein | 0.5g |
| Glucose | 0.5g |
| Starch | 0.5g |
| K2HPO4 | 0.03g |
| MgSO4, anhydrous | 0.024g |
| Sodium pyruvate | 0.3g |
| Agar | 15g |
| Water | 1000ml |
After sterilisation in autoclave, pH 7.2±0.2 (adjusted with K2HPO4 or KH2PO4).
TSA (Bacto Tryptic Soy Agar, Difco=CASO Agar, medium B).
From the point of view of this guideline, the bacterial endotoxin test applies to the determination or quantification of endotoxins from gram-negative bacteria. At present, the method based on the use of circulating horseshoe crab Limulus polyphemus amoebocyte lysate as reagent should be used (LAL test).
When the LAL reagent is put in contact with endotoxin-containing solutions in the presence of divalent cations, an enzymatic reaction takes place that converts clottable protein (coagulogen) into a gel (coagulin). The speed of this reaction depends on the concentration of endotoxin, the pH and the temperature. With this method, a semi-quantitative determination of the presence of endotoxin is obtained. The endpoint determination of the reaction is done by direct comparison with a control or reference endotoxin. In all tests for endotoxins, an international reference endotoxin (E. coli 0113 H10K) is used which serves as a standard sample for different determinations. The results are expressed as EU (endotoxin unit) or IU (International Unit), 1IU being equivalent to 1 EU (1IU=1 EU).
Other spectrophotometric methods (turbidimetric and chromogenic) have been developed which, via the LAL test, allow quantitative estimates of endotoxin content. These methods are based on the appearance of colour after degradation of a synthetic peptide that contains a chromophore.
The detection and quantification of ET shall be performed with a LAL test, in line with the technical recommendations of the European Pharmacopoeia and European Best Practice Guidelines for haemodialysis (Part 1) NDT 17, suppl.7, 2002. The techniques generally used are: (1) Gel-Clot method (Mallinckrodt® Inc.), semi-quantitative method; (2) Turbidimetric technique (Endosafe®, Charles River Laboratories Inc.), kinetic method; (3) kinetic chromogenic technique (Endosafe®, Charles River Laboratories Inc.), the most sensitive. The first two methods are acceptable for testing purified water and standard dialysate, but for highly purified water and ultrapure dialysate, the third shall be used.
The FDA also published a LAL Test Guideline.1
The samples for ET shall be collected as discussed in Section 6.2: a 5ml sample in a special pyrogen-free plastic tube without adsorptive capacity for ET. Samples shall be stored frozen and processed as soon as possible.
It is important to remember that there are other bacterial components (both bacterial membrane and bacterial DNA) which are not detected by standard methods, i.e. LAL, and can induce activation of immunocompetent cells. Some of these products are released into the circulation after bacterial lysis, while others, like exotoxins, are secreted. Many of these products can diffuse through the dialysis membrane due to their low molecular weight, the majority below 10kD.
Disinfection systems shall be effective for the inactivation or removal of microflora.
The frequency of disinfection is very important and must be programmed more for prevention than for the removal of contamination.
The disinfection shall reach all elements of the system. This includes the reverse osmosis membranes (especially the clean side), the distribution piping, the input lines to dialysis machines (located between the circuit and the machines), and the dialysis machine itself (which has its own circuit and disinfection program).
The disinfection procedure, when applied with appropriate frequency and including the critical areas, shall be capable of minimising the effects of biological contamination.
UV lamps can be used for inactivation of planktonic species, but has little or no activity against any biofilm formed in the system.
It is important to always refer to the manufacturers’ recommendations and, once disinfected, the absence of contaminant residue in the circuits shall be confirmed before reuse.
An adequate disinfection strategy shall be preventive, and shall be adjusted based on the validation and revalidation results.
Germicidal systemsDisinfection may be carried out using heat or chemicals.
As discussed under pre-treatment, once chlorine and other oxidising systems are removed, the risk of bacterial contamination is high. The points at greatest danger of contamination are filters, the resins in the water softeners and deionisers and the activated charcoal filter.
There is also a risk of contamination in the tanks and the distribution circuit, especially if there are dead zones out of circulation.
The following are used to combat this:
- 1.
Injection of chlorine at the beginning of pre-treatment: this is done by the permanent addition of sodium hypochlorite or hydrochloric acid to the system, to achieve a concentration of 0.3mg/l of free chlorine.
It should be remembered that the chlorine and other disinfectants may alter some types of reverse osmosis membranes, such as those made of polyamide.
- 2.
Submicron filters: block bacteria, 0.1μm.
- 3.
Ultraviolet radiation lamps1: capable of destroying all types of bacteria in their different states. The bactericidal effect depends on the lamp power, water purity, flow rate and exposure time. These parameters have to be well designed to be effective. The lamps have to be replaced periodically. The danger of this system is that, if the water is heavily contaminated, the massive destruction of bacteria can cause a massive release of endotoxins which may then reach the patient.
- 4.
Ozone disinfection: this gas is unstable, with a half life in aqueous media of 30minutes, and is a powerful oxidising agent. To remove the ozone, a more powerful and multi-frequency UV lamp, twice the capacity of that used as a germicide, is required; for a given flow, for converting the ozone to molecular oxygen.
Compared to other systems such as chlorination, this system is more powerful and has a better cost-benefit ratio2.
- 5.
Periodic and effective disinfection of the water treatment plant: we recommend more frequent disinfection in summer, using disinfectant and descaling agents such as peracetic/acetic acid, hydrogen peroxide and to a lesser extent, aldehydes.
Hypochlorites should only be used if the reverse osmosis membranes are compatible.
- 6.
Hot water disinfection3–6:
Important that the circuits recommended for heat sterilisation are those manufactured in cross-linked polyethylene (PEXA), acrylonitrile butadiene styrene (ABS plastic), PVDF (polyvinylidene fluoride), PTFE (Teflon) or pharmaceutical grade stainless steel.
A material can only properly be disinfected with heat if it is previously clean.
Hot water can be used to control bacterial growth in water storage and distribution or dialysis systems. The exposure time should be according to the manufacturer's instructions. The heater shall supply the required hot water at the right temperature for the necessary time to all points of the circuit. The effectiveness shall be monitored permanently by bacterial culture and endotoxin testing.
The ability of hot water to disinfect a distribution system depends on temperature and exposure time.
The concept, Ao, is a way of calculating the “dose of thermal energy necessary” to disinfect, based on different combinations of time and temperature.
One Ao equals one second at 80°C. (1 Ao=1 second at 80°C)
where T is the temperature in °C; z equals 10°C; and Δt is the time in seconds.However, the standard requirements in terms of the temperature and time parameters for a disinfection process should be based on additional parameters that are rarely taken into consideration: the frequency or the type of microorganism.
The frequency shall be programmed to prevent the formation of biofilm. We recommend that this shall be daily for the circuit, connection to the machine and ultrafilters, and weekly for the osmosis membranes. The required quality of water can often be maintained with one or two thermal disinfections per week.
The effectiveness of thermal disinfection depends on:
Disinfection time
Microbiological activity and type of microorganism
Frequency of disinfection
Temperature of the disinfection water
The dose of thermal disinfection to remove 99.999% of microorganisms and guarantee the efficiency of the thermal disinfection process anywhere in the facility is considered to be 12000 Ao.
Automated disinfection systems should be used in the treated-water distribution circuit; they may operate by heat or chemicals, or be mixed, and should include an endotoxin filter. Automated systems make it easier and safer to maintain microbiological standards.
We recommend periodically using hypochlorite (bleach) or some other commercial drain cleaner for the maintenance of the general drainage pipes in order to prevent or remove any deposits of chemical or organic matter.
Always be aware of the importance of compatibility between different disinfectants, detergents or descaling agents, in order to avoid toxicity complications, gases or even explosions; remembering that this can affect not only the circuits, but also the drains.
| Agent | Incompatibility with… | Dangerous with… |
|---|---|---|
| Acetic acid | Bases | Hypochlorite (bleach) Hydrogen peroxide (H202) |
| Citric acid | Hypochlorite Hydrogen peroxide Bases | |
| Hydrogen peroxide+Peracetic acid | Acid concentrates Alkali concentrates | Hypochlorite Aldehydes Phenol. Alcohols |
| Hypochlorite | Acids | Hydrogen peroxide Aldehydes Nitrates Phenol. Alcohols |
| Aldehydes (formaldehyde and glutaraldehyde) | Acids Ammonia Phenol | Hypochlorite Hydrogen peroxide Hydrochloric acid. Chlorhexidine |
| Phenol | Alkalis Aldehydes | Hypochlorite Hydrogen peroxide |
| Alcohols (ethanol-methanol) | Hypochlorite Hydrogen peroxide Potassium |
After any chemical disinfection, the substances used shall be cleared by rinsing and tests shall be performed to check for residual levels; for example, formaldehyde <3mg/l, bleach <0.1mg/l and ozone <0.1mg/l.
The following table compares the allowable levels of the elements to be monitored in purified water from different guidelines. We recommend that the main reference shall be the ISO-13959:2014. The chemical elements have been divided into three groups according to the criteria of these standards.
Maximum allowable levels of toxic chemical substances and dialysate electrolytes in water for dialysis. Values in mg/l.
| Contaminant | ISO-13959:20141 AAMI-13959:2014 | European Pharmacopoeia 4.32 | UNE standard 111-301-903 | Parametric value in drinking water, RD 140/20034 |
|---|---|---|---|---|
| Contaminants with documented toxicity in haemodialysis (first group) | ||||
| Aluminiuma | 0.01 | 0.01 | 0.01 | 0.2 |
| Total chlorine | 0.1 | 0.1 | – | – |
| Free chlorine | – | 0.5 | 0.5 | 1 |
| Combined chlorine (chloramines) | – | – | 0.1 | 2 |
| Copper | 0.1 | 0.1 | 0.1 | 2 |
| Fluoride | 0.2 | 0.2 | 0.2 | 1.5 |
| Lead | 0.005 | 0.005 | 0.005 | 0.01 |
| Nitrate as N | 2 | 2 | 2 | 50 |
| Sulfates | 100 | 50 | 100 | 250 |
| Zinc | 0.1 | 0.1 | 0.1 | – |
| Electrolytes normally included in the dialysate (second group) | ||||
| Calcium | 2 (0.05mmol/l) | 2 | 2 | – |
| Magnesium | 4 (0.15mmol/l) | 2 | 4 | – |
| Potassium | 8 (0.2mmol/l) | 2 | 8 | – |
| Sodium | 70 (3.0mmol/l) | 50 | 70 | 200 |
| Maximum levels of other toxic substances (third group) | ||||
| Antimonyb | 0.006 (0.005)a | 0.006 | – | 0.005 |
| Arsenic | 0.005 | 0.005 | 0.005 | 0.01 |
| Barium | 0.1 | 0.1 | 0.1 | – |
| Beryllium | 0.0004 | 0.0004 | – | – |
| Cadmium | 0.001 | 0.001 | 0.001 | 0.005 |
| Chromium | 0.014 | 0.014 | 0.014 | 0.05 |
| Mercury | 0.0002 | 0.0001 | 0.0002 | 0.001 |
| Selenium | 0.09 | 0.09 | 0.09 | 0.001 |
| Silver | 0.005 | 0.005 | 0.005 | – |
| Thallium | 0.002 | 0.002 | – | – |
| Other substances identified as toxic in dialysis | ||||
| Ammonium | – | 0.2 | 0.5 | |
| Chlorides | 50 | 250 | ||
| Heavy metals | 0.1c | 0.1 | – | |
We have taken the standard, UNE 111-301-90, as reference because, although outdated in several respects, it is still cited as Spanish regulation. It corresponds to the AAMI 1981.
The maximum level of these same substances in drinking water or feed or raw water are indicated as reference; ISO-13959:2014 indicates the importance of this as feed water; other national guidelines also specify this testing.5
ISO-13959:2014 provides some exceptions, such as the elements that it may not be possible to analyse in the third group,1 which do not need to be taken into consideration in Spain due to the technology in the treatment of feed water, the regulations on the water to be treated and the analytical capabilities of the laboratories.
The testing methods are described in ISO-13959:2014 and European Pharmacopoeia 4.3 01/2003:1167, Page 3049.
Considerations concerning some of the elementsChlorine and chloraminesChlorine is added to potable water as a bactericide because it is a strong oxidising agent. This function is performed by free chlorine, which quickly dissolves. The way to maintain stable levels of free chlorine is the formation of chloramines, mono-, di- and trichloride nitrogen compounds, which release the chlorine slowly. Chloramines are able to pass through most water treatment systems, including reverse osmosis. There are essentially two systems for removing them from water: reaction with activated charcoal; and reaction with sodium bisulfite. The choice of one system or another depends on the characteristics of the water to be treated and the pH these reactions cause. In the case of purified water for HD, we recommend activated charcoal as it is easier to maintain and replace and it removes other organic products, the proper maintenance and regular renewal is essential. If small amounts of chloramines cross into the blood, they cause oxidant effects, the most important being haemolysis. Chloramines are difficult to measure so concentrations are usually estimated as the difference between total chlorine and free chlorine.6 When measured using this method, the allowable levels should be at least less than 0.06mg/l for total chlorine and less than 0.05mg/l for chloramines.6
Total chlorine is the sum of free chlorine and chloramines, these in turn being the sum of monochloramines, dichloramines and nitrogen trichloride.
AluminiumAnother complex issue with dialysate is its aluminium content. The aluminium in water is present as ions associated with salts and in colloidal form, bound to organic material. Depending on the pH, the ion form can vary from a trivalent cation to a complex anion. Softeners only remove the cation forms. Colloidal aluminium is not removed by electro-deionization systems (DI); the only process with the capacity to remove it is reverse osmosis (RO). Aluminium is sometimes added to water as flocculant for organic matter, so it can be in very high concentrations. In such situations, the only way to achieve optimal levels in the dialysate is to work in series with two RO or DI-RO.7
We know that the balance of aluminium during dialysis is established between free or ultrafiltrable plasma aluminium, 5–10% of the total, and the aluminium in the dialysate and, if we want to create a distinct negative balance, maintaining Al concentrations in blood below 30–50μg/l, we have to maintain the concentrations in dialysate below 5μg/l.8 The level of aluminium in the treated water can be a reference for the proper operation of the water treatment system; knowing that the aluminium content in the water to be treated is a maximum of 200μg/l and that water treatment with two-stage osmosis is capable of retaining>98% of ions means that aluminium levels can be maintained constantly below 5μg/l.
Measuring substances such as aluminium requires a precise methodology, the use of non-metallic needles and special tubes, and preventing contamination of any kind.
IronIron is not included among the contaminants, but is particularly important because of its effect on osmosis membranes; it can foul membranes, affecting normal functioning, as it is not removed in the membrane reject. The maximum level in drinking water is 200μg/l, but from time to time and due to changes in the facilities, levels can become higher and then cause the above problems. Iron levels should therefore be monitored both in the water to be treated and before entering the osmosis system.1,9
Monitoring of silt density index (SDI)Measurement of SDI is useful in feed and pre-treated water and even at some intermediate point of the pre-treatment. It provides information on the quality of the water delivered to the reverse osmosis system, the capacity of the pre-treatment to retain particles in suspension, and the quality of the feed or raw water. Measurement of SDI is particularly recommended in the event of premature saturation of the RO system or alterations in any of the pre-treatment components. Manufacturers of reverse osmosis membranes and water treatment equipment indicate a maximum of SDI of 5 pre RO (some manufacturers lower this to 3). Changing SDI conditions in the feed water supply can result in the pre-treatment being unable to reduce it to the required levels. Monitoring the SDI becomes an effective tool with a view to implementing measures to protect the RO system equipment and prevent more major problems.
As mentioned in Section 4 of this guideline, as a starting point to be able to produce a quality dialysis fluid, first and foremost is the necessity of having a water supply that complies with RD 140/2003 on the quality criteria of water for human consumption and RD 865/2003, which establishes the health and hygiene criteria for the prevention and control of Legionnaires’ disease. Both decrees set out the health criteria for the quality of potable water, construction products used in the distribution and storage system and the actions necessary for maintenance and conservation of the facilities (see Appendix 1).
Annex I of RD 140/2003 sets out the maximum allowable level for the different chemical and microbiological parameters in drinking water. Annex II describes the UNE-EN standards and the Order SSI/304/2013, of 19 February, which derogate Order SAS/1915/2009, of 8 July, concerning the substances used in the treatment of drinking water. Regulations for Reference Laboratories, methods to be used for the determination of the different parameters and the testing frequency are all specified in the other Annexes. In addition, an information system on areas of supply and quality control of drinking water, the Sistema de Información Nacional de Agua de Consumo (SINAC) [Spanish Information System for Drinking Water] (http://sinac.msc.es) has been set up, through which national annual reports for public information are compiled which contain a large amount of information on the water, including its origin (groundwater, desalination plants, reservoirs, etc.), treatment process, disinfection system, microbiological and chemical analyses, etc.
Despite all the regulation, water used for dialysis shall meet much more demanding criteria in order to prevent complications and disease resulting from microbiological and chemical contaminants in water from the public supply. The level of purity required for dialysate has steadily increased with the introduction of haemodialysis using high-flux membranes and especially the convective techniques in which the dialysate itself is infused.
The first Spanish regulation for water treatment for haemodialysis was the UNE 111-301-90 standard. The 1st edition of this guideline published in 2004 set the standard to follow in terms of water quality for haemodialysis in Spain and even outside Spain. Many of Spain's autonomous regions have used this as a basis for their technical specification documents and agreements for haemodialysis, although each with its distinctive features. In 2011, the Ministry of Health, Social Policy and Equality published a review of standards and recommendations for renal replacement therapy (haemodialysis) units, in which all aspects of water treatment for haemodialysis were also based on the 1st edition of this guideline.
The international standard ISO, which is very close to our guideline on the cut-off values for microbiological contaminants, is gaining increasingly more followers and it is being used in some haemodialysis units here in Spain.
The different references for the standards and recommendations are provided below, grouped by product type. It is important to note that the reference standard is the ISO 2014 and, in parallel, with identical nomenclature, the American AAMI recommendations.
Water for public consumption, potable water or feed water:
- A.
Regulations:
- –
ROYAL DECREE 140/2003, of 7 February, establishing health criteria for the quality of water for human consumption.
- –
ROYAL DECREE 865/2003, of 4 July, establishing health and hygiene criteria for the prevention and control of Legionnaires’ disease.
- –
Water for haemodialysis and dialysate:
- A.
Regulations and recommendations:
- –
Royal Decree 414/1996, of 1 March, regulating medical devices.
- –
UNE (Una Norma Española) [A Spanish Standard]. AENOR (Asociación Española de NORmalización) [Spanish Association for Standardisation].
- •
UNE 111301:1990. Characteristics of water used in haemodialysis.
- •
UNE EN 13867:2003+A1:2009. Concentrates for haemodialysis and related therapies.
- •
UNE for ISO 11663:2014, (BOE-A-2015-5655.pdf)
- •
- –
Renal Replacement Unit, Standards and Recommendations, Reports, Studies and Research 2011. Ministerio de Sanidad, Política Social e Igualdad. http://www.msssi.gob.es/organizacion/sns/planCalidadSNS/docs/EERR/UDE.pdf
- –
Real Farmacopea Española [Royal Spanish Pharmacopoeia], 5th Ed. (Feb 2015)
- –
European Pharmacopoeia. 8th Ed. (2014) https://www.edqm.eu/en/european-pharmacopoeia-8th-edition-1563.html
• Concentrated solutions for haemodialysis 2376 • Concentrates for injections or infusions 797 • Conductivity (2.2.38.) 59 • Haemodiafiltration and haemofiltration, solutions for 2378 • Haemodialysis, concentrated solutions for 2376 • Haemodialysis solutions, concentrated, water for diluting 2375 • Haemodialysis, solutions for 2376 • Haemofiltration and haemodiafiltration, solutions for 2378 • Solutions for haemodialysis 2376 • Solutions for haemodialysis, concentrated, water for diluting 2375 • Solutions for haemofiltration and haemodiafiltration 2378 • Solutions for peritoneal dialysis 2994 • Water for diluting concentrated haemodialysis solutions 2375 • Water for injections 3555 • Water, highly purified 3559 • Water, purified 3561 - –
AAMI (Association for the Advancement of Medical Instrumentation). http://www.aami.org
Using the same nomenclature as ISO standards since 2011. Required for “Centers for Medicare and Medicaid Services (CMS)”
- •
ANSI/AAMI/ISO 11663:2014 Quality of dialysis fluid for hemodialysis and related therapies.
- •
ANSI/AAMI/ISO 13958:2014 Concentrates for hemodialysis and related therapies.
- •
ANSI/AAMI/ISO 13959:2014 Water for hemodialysis and related therapies.
- •
ANSI/AAMI/ISO 23500:2014 Guidance for the preparation and quality management of fluids for hemodialysis and related therapies.
- –
ISO. (International Organization Standardization). http://www.iso.org
International standards with which compliance is voluntary.
- •
ISO 11663 2014 Quality of dialysis fluid for haemodialysis and related therapies
- •
ISO 13958 2014 Concentrates for haemodialysis and related therapies
- •
ISO 13959 2014 Water for haemodialysis and related therapies
- •
ISO 23500 2014 Guidance for the preparation and quality management of fluids for haemodialysis and related therapies
- •
- B.
Guidelines:
- –
Guideline for Dialysate Quality (GDQ). 2004.
http://www.senefro.org/modules/webstructure/files/gua_calidad_agua_mar06.pdf?check_idfile=1368
- –
UK Renal Association and Association of Renal Technologist. Guideline on water treatment facilities, dialysis water and dialysis fluid quality of haemodialysis and related therapies. 2012
- –
European Best Practice Guidelines for Haemodialysis (part 1) Nephrol. Dial. Transplant. 2002:17 (Suppl. 7): 45–62
- –
The following do not cover water for HD or dialysate:
- •
Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines
- •
Kidney Disease Improving Global Outcomes (KDIGO) guidelines
- •
Canadian Society of Nephrology (CSN) guidelines
- •
Caring for Australasian with Renal Impairment (CARI) guidelines
- •
- –
Medical electrical equipment (MD)
This is a non-implantable medical device (NIMD) and as such, subject to European and national regulations (i.e. RD 1591/2009, Medical Devices Directive, 93/42/CEE, ISO13485, IEC 60601.1:2015), whose general requirements in terms of design and manufacturing must be met to be in compliance and which are monitored by the Agencia Española del Medicamento y Productos Sanitarios (AEMPS) [Spanish Agency of Medicines and Medical Devices] among other authorities.
- -
http://www.aemps.gob.es/productosSanitarios/prodSanitarios/home.htm
- -
http://www.aenor.es/aenor/normas/normas/fichanorma.asp?tipo=N&codigo=N0051276&pdf=
- -
http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1993L0042:20071011:EN:PDF
- -
https://www.boe.es/diario_boe/txt.php?id=BOE-A-2009-17606
- -
http://www.iso.org/iso/home/store/catalogue_ics/catalogue_detail_ics.htm?csnumber=65529
By definition, MEDICAL DEVICE is any instrument, apparatus, appliance, software programme or other article, whether used alone or in combination, intended by the manufacturer to be used in human beings for the purpose of diagnosis, prevention, monitoring, treatment or alleviation of disease.
Dir. 2007/47http://www.boe.es/doue/2007/247/L00021-00055.pdf
Haemodialysis equipment:
- –
UNE (Una Norma Española) [A Spanish Standard]. AENOR (Asociación Española de NORmalización) [Spanish Association for Standardisation].
- •
UNE-EN 60601-2-16 CORR:2000. Medical electrical equipment - Part 2-16: Particular requirements for the safety of haemodialysis, haemodiafiltration and haemofiltration equipment.
- •
UNE-EN 60601-2-16:1999. Equipos electromédicos. Part 2-16: Particular requirements for the safety of haemodialysis, haemodiafiltration and haemofiltration equipment.
- •
Renal Replacement Unit, Standards and Recommendations, Reports, Studies and Research 2011. Ministerio de Sanidad, Política Social e Igualdad” http://www.msssi.gob.es/organizacion/sns/planCalidadSNS/docs/EERR/UDE.pdf
- •
ISO 26722 2014 Water treatment equipment for haemodialysis applications and related therapies
- •
ANSI/AAMI/ISO 26722:2014 Water treatment equipment for hemodialysis applications and related therapies.
- •
Please cite this article as: Pérez-García R, García Maset R, Gonzalez Parra E, Solozábal Campos C, Ramírez Chamond R, Martín-Rabadán P, et al. Guía de gestión de calidad del líquido de diálisis (LD) (segunda edición, 2015). Nefrologia. 2016;36:e1–e52.