Physical exercise may offer multiple benefits to patients with chronic kidney disease (CKD). However, it was not traditionally recommended because of the possibility of impairing renal function and increasing proteinuria. The objective of this study is to review the clinical trials on physical exercise in patients with CKD and describe its effect on the progression of kidney disease and other factors associated. Randomised clinical trials (RCT) comparing an intervention that included an exercise component with a control group without physical exercise in non-dialysis patients with CKD from 2007 to 2018 in English and Spanish were included. PubMed, Scopus, Embase, Ovid (Medline) and PEDro databases were used for the search. Effects of physical exercise were summarised by the standardised mean difference (SMD). No differences were found in glomerular filtration rate or proteinuria between the intervention group and the control group: SMD −0.3 (p=0.81); SMD 26.6 (p=0.82). Positive effects were obtained on peak oxygen consumption: SMD 2.5 (p<0.001), functional capacity: SMD 56.6 (p<0.001), upper limb strength: SMD 6.8 (p<0.001) and haemoglobin: SMD 0.3 (p=0.003). An improvement on the quality of life was also evident using the KDQOL-36 survey: SMD 3.56 (p=0.02) and the SF-36 survey: SMD 6.66 (p=0.02). In conclusion, the practice of low-intensity physical exercise routinely has no negative impact on renal function. On the contrary, it improves aerobic and functional capacity, impacting positively on the quality of life.
El ejercicio físico podría ofrecer múltiples beneficios al paciente con enfermedad renal crónica (ERC). No obstante, tradicionalmente no se recomendaba por la posibilidad de deteriorar la función renal y aumentar la proteinuria. El objetivo del estudio es revisar los ensayos sobre ejercicio en pacientes con ERC y describir su impacto sobre la progresión de la enfermedad renal y otros factores asociados. Se seleccionaron ensayos clínicos aleatorizados desde 2007 a 2018, en inglés y en español, que compararan un grupo intervención con un componente de ejercicio con un grupo control sin ejercicio físico en pacientes con ERC en prediálisis. Para la búsqueda se emplearon las bases de datos PubMed, Scopus, Embase, Ovid (Medline) y PEDro. Los efectos del ejercicio sobre las variables analizadas se resumieron calculando la diferencia de medias estandarizada (DME). No se encontraron diferencias en el filtrado glomerular ni en la proteinuria entre el grupo intervención y el grupo control (DME: −0,3; p=0,81; DME: 26,6; p=0,82). Se obtuvieron efectos positivos sobre el consumo pico de oxígeno (DME: 2,5; p<0,001), la capacidad funcional (DME: 56,6; p<0,001), la fuerza en miembros superiores (DME: 6,8; p<0,001) y la hemoglobina (DME: 0,3; p=0,003). También se evidenció mejoría sobre la calidad de vida usando los cuestionarios KDQOL-36 (DME: 3,56; p=0,02) y SF-36 (DME: 6,66; p=0,02). En conclusión, la práctica de ejercicio de forma rutinaria y a baja intensidad no tiene impacto negativo sobre la función renal. Por el contrario, mejora la capacidad aeróbica y funcional, repercutiendo positivamente en la calidad de vida.
Chronic kidney disease (CKD) represents a state of significant oxidative stress, proinflammation and malnutrition. This leads to the accumulation of metabolic waste products and alterations in homeostasis which affect many target organs, including the cardiovascular system, which gradually leads to reduced physical capacity and increased mortality.1–3 Alterations also occur in the locomotor system, such as osteoporosis and loss of muscle mass.4 Sarcopenia is present from the early stages of the disease and its prevalence increases in the more advanced stages, which is correlated with higher mortality and disability rates, and an increased risk of falls, fractures and hospitalisation.5–7
Traditionally, doing physical activity was not recommended to patients with CKD due to the possibility of impairing kidney function and increasing proteinuria.8 Today it is known that a sedentary lifestyle can be both the cause and the consequence of kidney disease progression, meaning that physical activity is reduced as glomerular filtration rate decreases. This is a key point which should be acted on, as it is a modifiable factor with a clear impact on the survival of these patients.9,10
Within the health recommendations, it is advised to include regular physical activity from the initial stages, which improves physical and psychological condition at the same time as reducing mortality and offering a greater quality of life.4,10 However, despite the fact that the benefits of exercise in CKD patients seem evident, it is still not clear which parameters related to CKD are improved by physical activity or which is the best training programme. Nor is it included routinely within the integrated management of these patients.9–11
The main objective of this paper is to systematically review the studies on physical activity carried out in CKD patients who do not yet require renal replacement therapy (RRT) and to describe the impact of physical activity on kidney disease progression. As a secondary objective, the effect of physical activity on cardiovascular risk factors, physical condition, quality of life and mortality are reviewed.
Material and methodsData source and search strategyThe databases PubMed, Scopus, Embase, Ovid (MEDLINE) and PEDro were used for the search. The following keywords were entered: “exercise”, “physical activity”, “physical exercise”, “physical function”, “resistance training”, “quality of life”, “randomised controlled trial” and “chronic kidney disease”. All the above terms and their combinations were included.
Selection criteriaRandomised clinical trials (RCT) published between 2007 and 2018, both in English and Spanish, which compared an intervention that included an exercise component with a control without exercise in CKD patients not on dialysis were selected. Studies were included if authors used words such as “random” to describe the method of assigning subjects to groups. The review and inclusion of the articles cited was carried out by one single investigator.
Opinion articles, communications at congresses and single cases were excluded. Likewise, trials with patients on haemodialysis, peritoneal dialysis or kidney transplant patients, trials which included patients with a history of heart conditions (decompensated heart failure, unstable angina or arrhythmias) articles with lung disease patients (chronic obstructive pulmonary disease, bronchial asthma, chronic bronchitis), articles which included patients with degenerative neurological conditions or disorders of the locomotor system and articles in languages other than those mentioned above were excluded.
The interventions were classified according to the nature of the main phase of the exercise: aerobic interventions or aerobic interventions combined with load exercises. No restrictions were made on the frequency or intensity of the exercise, although a minimum duration of 12weeks was required.
Statistical analysisThe data analysis was carried out using the RevMan Analysis program, version 5.3. The results were presented as mean and standard deviation with a 95% confidence interval (CI) for the continuous variables, which was summarised based on the calculation of the standardised mean difference (SMD). In the case of qualitative variables, the results were expressed as frequencies and odds ratios (OR). As a mean, the difference between the means of the pre- and post-intervention variables were used, using the standard deviation following the intervention. The results are presented using “forestplot”.
Heterogeneity among the trials was assessed with the I2 and X2 heterogeneity tests. Values of I2>50% and X2<0.1 were considered to be indicative of significant heterogeneity. In this case, the random effects model was used for the meta-analysis. A p-value of <0.05 was considered statistically significant.
Quality assessmentOnce the search had been completed, a critical review was carried out to rule out the studies which should not be included in the review (not related to the objectives and/or methodological criteria). To assess the methodological quality of the RCTs, we used the Jadad scale, which scores clinical trials from 0 to 5, considering them to be poor quality if the score is less than 3.12
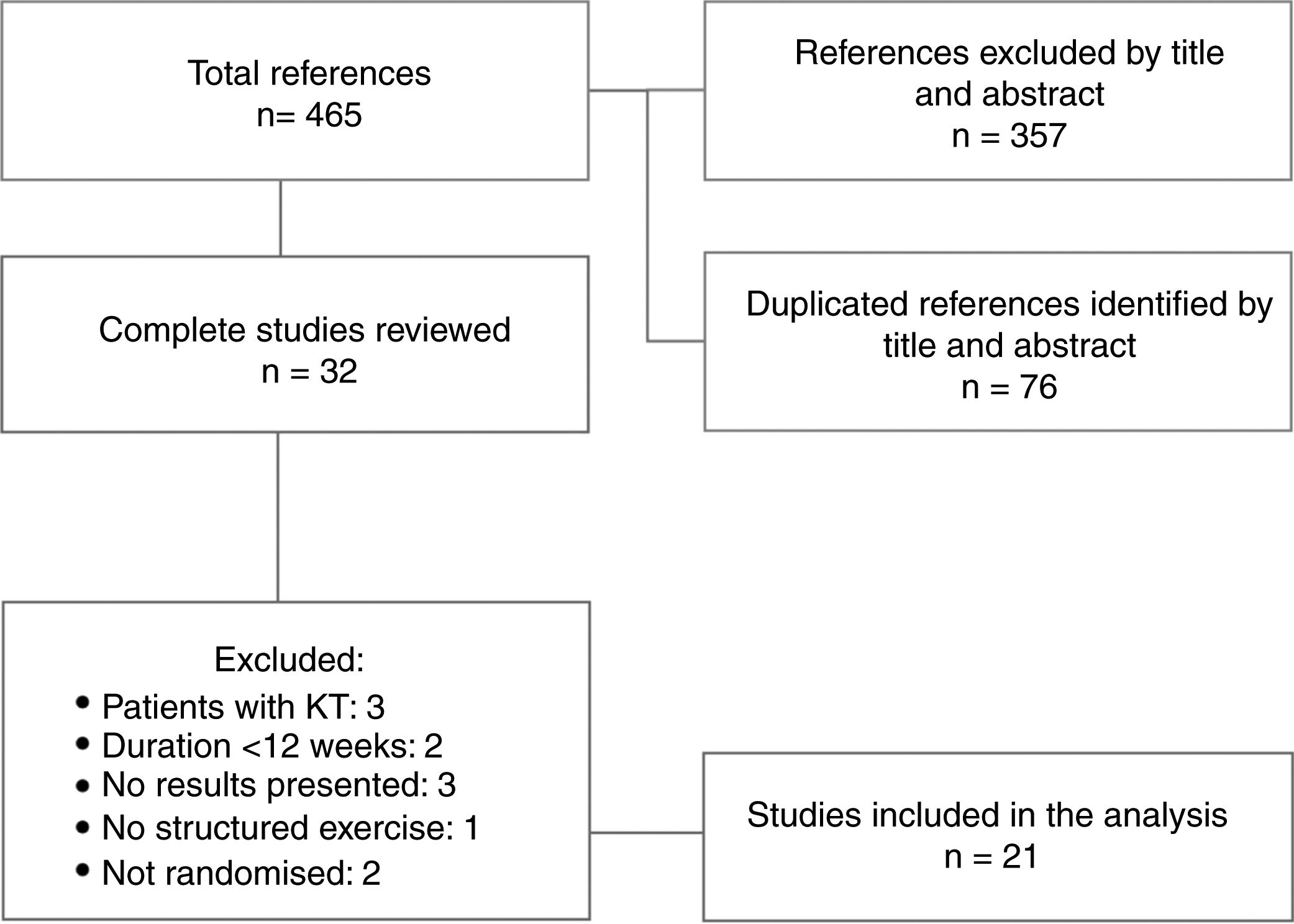
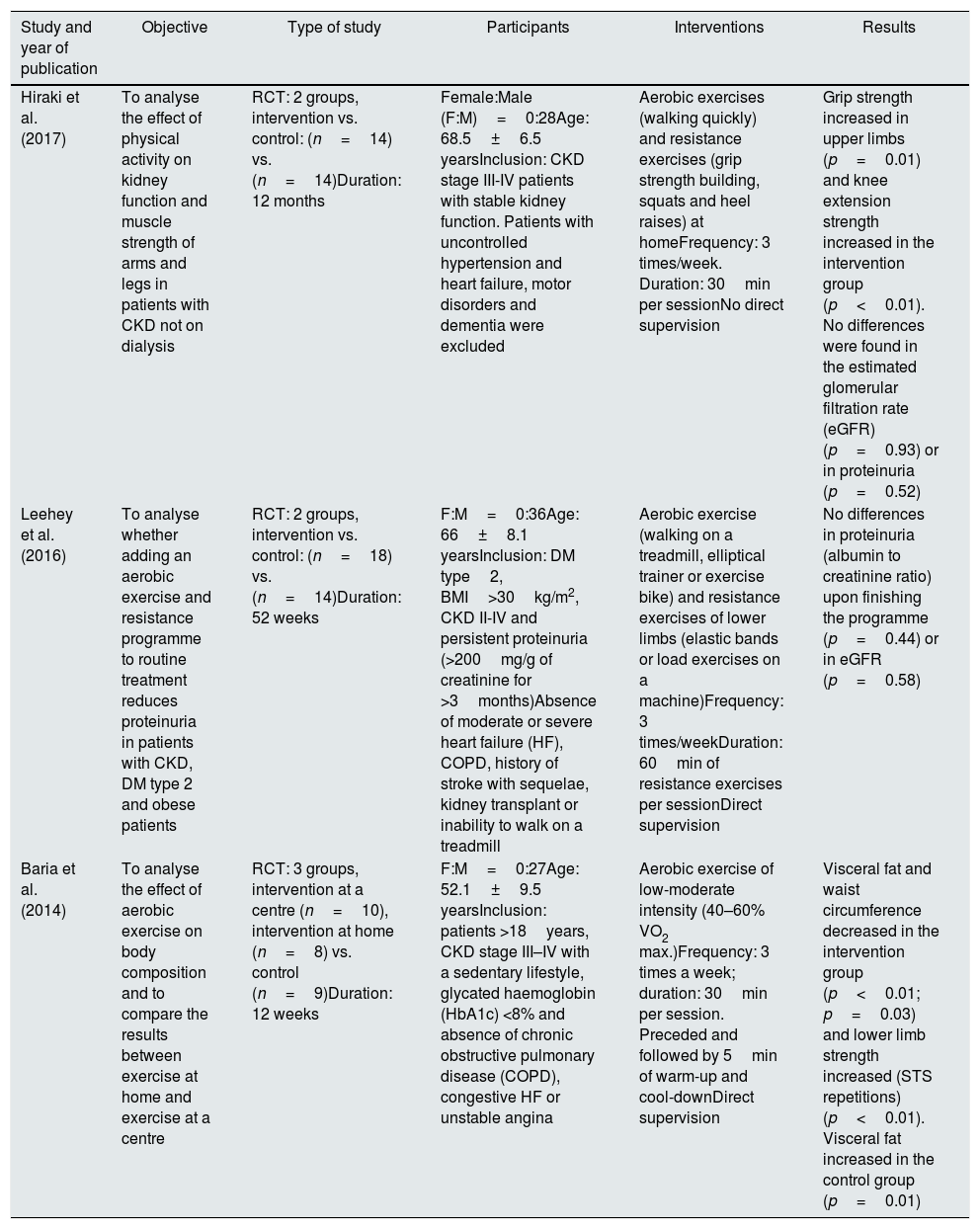
ResultsDescription of the studiesA total of 465 references with potentially useful reports were found: 219 in PubMed, 89 in Scopus, 107 in Embase, 50 in Ovid (MEDLINE) and none in PEDro. The number of articles ruled out was 433. After adjusting for records duplicated from the title and abstract, 32 reports were left to review completely. In the end, 21 studies were included for the analysis. The search for the selection of studies included and the reasons for exclusion are shown in Fig. 1. Table 1 summarises the main aspects of the works included.
Characteristics of the studies included.
| Study and year of publication | Objective | Type of study | Participants | Interventions | Results |
|---|---|---|---|---|---|
| Hiraki et al. (2017) | To analyse the effect of physical activity on kidney function and muscle strength of arms and legs in patients with CKD not on dialysis | RCT: 2 groups, intervention vs. control: (n=14) vs. (n=14)Duration: 12 months | Female:Male (F:M)=0:28Age: 68.5±6.5 yearsInclusion: CKD stage III-IV patients with stable kidney function. Patients with uncontrolled hypertension and heart failure, motor disorders and dementia were excluded | Aerobic exercises (walking quickly) and resistance exercises (grip strength building, squats and heel raises) at homeFrequency: 3 times/week. Duration: 30min per sessionNo direct supervision | Grip strength increased in upper limbs (p=0.01) and knee extension strength increased in the intervention group (p<0.01). No differences were found in the estimated glomerular filtration rate (eGFR) (p=0.93) or in proteinuria (p=0.52) |
| Leehey et al. (2016) | To analyse whether adding an aerobic exercise and resistance programme to routine treatment reduces proteinuria in patients with CKD, DM type 2 and obese patients | RCT: 2 groups, intervention vs. control: (n=18) vs. (n=14)Duration: 52 weeks | F:M=0:36Age: 66±8.1 yearsInclusion: DM type2, BMI>30kg/m2, CKD II-IV and persistent proteinuria (>200mg/g of creatinine for >3months)Absence of moderate or severe heart failure (HF), COPD, history of stroke with sequelae, kidney transplant or inability to walk on a treadmill | Aerobic exercise (walking on a treadmill, elliptical trainer or exercise bike) and resistance exercises of lower limbs (elastic bands or load exercises on a machine)Frequency: 3 times/weekDuration: 60min of resistance exercises per sessionDirect supervision | No differences in proteinuria (albumin to creatinine ratio) upon finishing the programme (p=0.44) or in eGFR (p=0.58) |
| Baria et al. (2014) | To analyse the effect of aerobic exercise on body composition and to compare the results between exercise at home and exercise at a centre | RCT: 3 groups, intervention at a centre (n=10), intervention at home (n=8) vs. control (n=9)Duration: 12 weeks | F:M=0:27Age: 52.1±9.5 yearsInclusion: patients >18years, CKD stage III–IV with a sedentary lifestyle, glycated haemoglobin (HbA1c) <8% and absence of chronic obstructive pulmonary disease (COPD), congestive HF or unstable angina | Aerobic exercise of low-moderate intensity (40–60% VO2 max.)Frequency: 3 times a week; duration: 30min per session. Preceded and followed by 5min of warm-up and cool-downDirect supervision | Visceral fat and waist circumference decreased in the intervention group (p<0.01; p=0.03) and lower limb strength increased (STS repetitions) (p<0.01). Visceral fat increased in the control group (p=0.01) |
| Study and year of publication | Objective | Type of study | Participants | Interventions | Results |
|---|---|---|---|---|---|
| Leehey et al. (2009) | To analyse the effects of physical activity on the cardiovascular system and proteinuria in diabetic patients | RCT: 2 groups, intervention vs. control: (n=7) vs. (n=6)Duration: 24 weeks | F:M =0:13Age: 66 (55–81)Inclusion: patients with CKD stage II-IV, DM type2, BMI>30 with persistent proteinuria (>200mg/g of creatinine for >3months) | Aerobic exercise (walking) of moderate intensity (50–60% VO2 max.)Frequency: 3 times/week. Duration: 30min per session. Preceded and followed by 5min of warm-up and cool-downWithout direct supervision | Aerobic capacity improved in the intervention group (greater exercise duration) (p<0.05). No significant changes in the blood pressure levels at rest or in 24-h proteinuria (p>0.05) |
| Gomes et al. (2017) | To analyse the effect of aerobic exercise on bone turnover markers of overweight patients with CKD | RCT: 2 groups, intervention vs. control: (n=24) vs. (n=14)Duration: 24 weeks | F:M=12:27Age: 55.5±8.3 yearsInclusion: patients >18years, CKD stage III–IV, BMI>25kg/m2. Absence of COPD, congestive heart failure or unstable angina | Low-moderate intensity aerobic exercise (40–60% VO2 max.) without supervision (walking in parks or in the street), or with direct supervision (on a treadmill)Frequency: 3 times/weekDuration: 30min per session. Increase of 10min per session every 4 weeks up to 8th weekSupervision/no supervision | Alkaline phosphatase was the only bone turnover marker which increased in the intervention group (p<0.05)Functional capacity improved in the intervention group (6MWT) (p<0.001), as did aerobic capacity (VO2 max.) (p<0.001)Kidney function showed no difference between both groups (CKD EPI) (p=0.209) |
| Van Craenenbroeck et al. (2015) | To analyse whether an aerobic training programme improves peripheral endothelial function | RCT: 2 groups, intervention vs. control: (n=25) vs. (n=23)Duration: 12 weeks | F:M=18:22Age: 53.18±13.0 yearsInclusion: CKD stage III-IV patients and without established cardiovascular disease (coronary artery disease, peripheral vascular disease or cerebrovascular disease) | Aerobic exercise programme (cycling) at moderate intensity (90% of the maximum heart rate)Frequency: 4 sessions/dayDuration: 10min each sessionPartial supervision | No differences in vascular function in vivo (dilatation measured by brachial artery flow) (p=0.900). Aerobic capacity improved in the intervention group (VO2 max.) (p<0.001) |
| Study and year of publication | Objective | Type of study | Participants | Interventions | Results |
|---|---|---|---|---|---|
| Aioke et al. (2015) | To analyse the benefits of a physical activity programme at home in CKD patients not on dialysis who are overweight | RCT: 2 groups, intervention vs. control: (n=15) vs. (n=14)Duration: 48 weeks | F:M=27:35Age: 55.1±11.6 yearsInclusion: CKD stage III-IV patients with stable kidney function and who had diabetes and/or hypertension as a cause of their kidney disease | Aerobic exercise at home (walking in the park, the street, etc.)Frequency: 3 times/week. Duration: 30min per session. Increase of 10min per session every 4 weeks up to 8th week. Preceded and followed by 5min of warm-up and cool-downNo direct supervision | Functional capacity improved in the intervention group (6MWT) (p=0.028), which also developed more upper limb and lower limb strength (bicep curl rep and STS repetitions) (p<0.001, p<0.001) as well as improved lung capacity (maximum voluntary ventilation) (p=0.005)Reduced SBP and DBP (p=0.012, p=0.038) and improved kidney function (CKD EPI) (p=0.046) |
| Aioke et al. (2018) | To analyse the benefits of a physical activity programme at home or at a centre in CKD patients not on dialysis who are overweight | RCT: 2 groups, intervention vs. control: (n=25) vs. (n=15)Duration: 24 weeks | F:M=15:25Age: 55.5±8.3 yearsInclusion: Patients aged 18–70 years, CKD stage III-IV with stable kidney function and BMI>25kg/m2 | Aerobic exercise of low-moderate intensity (40–60% VO2 max.)Frequency: 3 times a week; duration: 30min per session. Increase of 10min per session every 4 weeks up to 8th week. Preceded and followed by 5min of warm-up and cool-downDirect supervision | Functional capacity improved in the intervention group (6MWT) (p<0.05), which also developed more upper limb and lower limb strength (bicep curl reps and STS repetitions) (p<0.05, p<0.05). Improvement in perceived quality of life and sleep quality (measured by the SF-36 scale) (p<0.05) |
| Study and year of publication | Objective | Type of study | Participants | Interventions | Results |
|---|---|---|---|---|---|
| Howden et al. (2015) | To analyse the efficacy, adherence and safety of a physical activity programme with a phase at a centre followed by another phase at home in CKD patients | RCT: 2 groups, intervention vs. control: (n=36) vs. (n=36)Duration: 12 weeks | F:M=18:22Age: 61.1±9.1 yearsInclusion: patients with CKD stage III-IV and one or more uncontrolled cardiovascular risk factors (HTN, BMI, glycaemic control, etc.) | Aerobic exercise (walking or jogging, cycling, rowing, etc.) of moderate intensity (RPE 13–15 points) and resistance training (weights or elastic bands)Frequency: not specified. Duration: 30min per sessionPartial supervision | Aerobic capacity (MET) (p<0.001) and functional capacity improved in the intervention group (6MWT) (p<0.001)Upper limb strength improved (grip strength) (p=0.003) and BMI decreased (p=0.07). No differences in eGFR (MDRD) (p=0.3) or in proteinuria (albumin to creatinine ratio) (p=0.9) |
| Howden et al. (2013) | To analyse the effect of physical activity on breathing capacity and the cardiovascular system in CKD patients | RCT: 2 groups, intervention vs. control: (n=36) vs. (n=36)Duration: 48 weeks | F:M=27:45Age: 61.1±9.1 yearsInclusion: patients with CKD stage III-IV and one or more uncontrolled cardiovascular risk factors (HTN, BMI, glycaemic control, etc.) | Aerobic exercise (treadmill, exercise bike, rowing, etc.) at moderate intensity (RPE of 11–13) and resistance exercisesFrequency: 2–3 times/weekDuration: 20–30min per session. Preceded and followed by a warm-up and cool-downPartial supervision | The intervention group improved aerobic capacity (VO2 max.) (p=0.004) and lost weight (p=0.02). The eGFR was similar in both groups (MDRD) (p=0.28) |
| Study and year of publication | Objective | Type of study | Participants | Interventions | Results |
|---|---|---|---|---|---|
| Gregory et al. (2011) | To assess changes in the insulin-like growth factor (IGF) and their related factors in patients with CKD in stages III and IV after a progressive physical training programme | RCT: 2 groups, intervention vs. no intervention:(n=10) vs. (n=11)Duration: 48 weeks | Age: 54.9±11.0 yearsInclusion: patients with CKD II-IV and on treatment with ACE inhibitors or ARBs. No patient had been on a physical training programme before the start of the study | Aerobic exercise (treadmill, exercise bike) at moderate intensity (50–60% VO2 max.) and nutritional adviceFrequency: 3 times/weekDuration: 55min per session. Preceded and followed by 5min of warm-up and cool-downDirect supervision | No significant differences in any of the IGF system components (p>0.05). Aerobic capacity (VO2 max.) (p=0.03) and exercise tolerance (total exercise time) (p<0.01) improved in the intervention group |
| Headley et al. (2017) | To analyse the relationship between aerobic exercise and the acute and chronic blood pressure response in CKD patients | RCT: 2 groups, intervention vs. control: (n=25) vs. (n=21)Duration: 16 weeks | F:M=13:30Age: 58.5±8.5 yearsInclusion: CKD stage III-IV patients with stable kidney function and who had diabetes and/or hypertension as a cause of their kidney disease | Aerobic exercise at moderate intensity (50–60% VO2 max.)Frequency: 3 times/weekDuration: 15–30min per session. Progressive increases up to 55min per sessionDirect supervision | The patients presented hypotension after one aerobic exercise session, without differences being found in their frequency between the control and intervention groups after 16 weeks of training (p=0.60)There were no differences in systolic or diastolic blood pressure (measured on an outpatient basis) between both groups at the end of the study (p=0.67, p=0.32) |
| Study and year of publication | Objective | Type of study | Participants | Interventions | Results |
|---|---|---|---|---|---|
| Headley et al. (2014) | To analyse the effect of short-term moderate intensity exercise on arterial stiffness in CKD stage III patients | RCT: 2 groups, intervention (n=25) vs. control (n=21)Duration: 24 weeks | F:M =16:30Age: 57.5±8.5 yearsInclusion: CKD stage III-IV patients with stable kidney function and who had diabetes and/or hypertension | Moderate-intensity aerobic exercise (50–60% VO2 max.)Frequency: 3 times/week. Duration: 30min per session. Progressive increase of time up to 55min of exercise per session. Preceded and followed by 5min of warm-up and cool-downDirect supervision | The VO2 peak (p=0.04) increased in the intervention group, although the pulse wave velocity did not change (p=0.8) |
| Headley et al. (2012) | To examine the effect of moderate-intensity training on kidney and vascular function in CKD patients | RCT: 2 groups, intervention vs. control: (n=10) vs. (n=11)Duration: 48 weeks | Age: 54.9±11.0 yearsInclusion: patients with CKD stage II-IV and aged 18–70. Patients were excluded if they had any contraindication to doing exercise | Moderate-intensity aerobic exercise (50–60% VO2 max.)Frequency: 3 times/weekDuration: 45min per session. Preceded and followed by 5min of warm-up and cool-downWithout direct supervision | Aerobic capacity (VO2 max.) improved in the intervention group (p<0.05), as did LDL cholesterol (p<0.05), with no changes in kidney function (MDRD). HR decreased (ambulatory and resting) (p<0.05) |
| Balakrishnan et al. (2010) | To analyse the effect of resistance training on the number of copies of mitochondrial DNA (mtDNA) of skeletal muscle and its association with the muscle phenotype (muscle mass and strength) | RCT: 2 groups, intervention (n=13) vs. control (n=10)Duration: 48 weeks | F:M =6:17Age: 64±10 yearsInclusion: adult patients with CKD stage III-IV with stable kidney function without treatment with renal replacement therapy | Resistance exercise (chest and leg press, knee extension and resistance training machines). At 80% intensity of one repetition maximumFrequency: 3 times/weekDuration: 35min per session. Preceded and followed by 5min of warm-up and cool-downDirect supervision | Significant increase in muscle mtDNA in the intervention group (p=0.001). The change in the number of copies of muscle mtDNA was positively correlated with an increase in the muscle fibre cross-sectional area for type I (r=0.56, p=0.01) and type II (r=0.46, p=0.05). |
| Study and year of publication | Objective | Type of study | Participants | Interventions | Results |
|---|---|---|---|---|---|
| Greenwood et al. (2015) | To analyse the effect of a moderate-intensity physical activity programme on kidney function and cardiovascular risk indices in CKD patients | RCT: 2 groups, intervention vs. control: (n=8) vs. (n=10)Duration: 12 months | F:M=3:15Age: 53.5±13.1 yearsInclusion: participants were included if they were >18 years and had evidence of a reduced GFR rate in the 12months before the intervention | Aerobic exercise (exercise bike) of moderate intensity (RPE 11 points) and resistance exercise (bicep curl, leg press, knee extension, etc.)Frequency: 3 times/week. Duration: 20–40min per sessionPartial supervision | The intervention group had less deterioration of eGFR (CKD-EPI) (p=0.02). They improved their pulse wave velocity (p=0.001), BMI (p=0.01) and weight (p=0.02) and waist circumference (p=0.003) |
| Tang et al. (2017) | To analyse the effect of a physical exercise programme on physical function, psychological sphere and quality of life in CKD patients | RCT: 2 groups, intervention vs. control: (n=42) vs. (n=42)Duration: 12 weeks | F:M=33:51Age: 43.1±14 yearsInclusion: patients aged 18–70. CKD stage I-III with stable kidney function | Aerobic exercise (walking, cycling or jogging) of moderate intensity (12–15 points on the RPE)Frequency: 3 times/week. Duration: 20–30min per sessionNo direct supervision | Improvement in functional capacity in the intervention group (6MWT) (p=0.001), as well as in lower limb strength (STS) (p<0.005)In addition, an improvement in patient quality of life was observed (KDQOL-36) (p=0.002) |
| Petcher et al. (2013) | To analyse the relationship of aquatic physical activity with mortality or initiation of dialysis in CKD patients | RCT: 2 groups, intervention vs. control: (n=7) vs. (n=9)Duration: 10 years | (F:M)=7:9Age: 49.7 (31–65) yearsInclusion: patients with CKD stages III-V not on dialysis | Low-intensity aquatic aerobic exercise (40–50% VO2 max.)Frequency: 2 times/weekDuration: 30min per session (plus 10min of warm-up and 10min of cool-down)Direct supervision | No differences between both groups in the final endpoint of the study (p=0.089). However, none of the members of the exercise group started dialysis or died in 10years. In the control group, 55% of patients required initiation of renal replacement therapy or passed away |
| Study and year of publication | Objective | Type of study | Participants | Interventions | Results |
|---|---|---|---|---|---|
| Rossi et al. (2014) | To analyse the benefit of physical activity on physical condition and health-related quality of life in CKD stage III-IV patients | RCT: 2 groups, intervention vs. control: (n=59) vs. (n=48)Duration: 12 weeks | F:M=51:56Age: 68.4±12.4 yearsInclusion: CKD stage III-IV patients with stable kidney function over the age of 18 | Aerobic exercise (treadmill and/or exercise bike) and resistance exercises (upper and lower limb extensions and free weight exercises)Frequency: 2 times/week. Duration: 60min per sessionDirect supervision | Functional capacity improved in the intervention group (6MWT) (p<0.001). Health-related quality of life (RAND-36) improved in the items related to the physical sphere (p<0.001) |
| Chen et al. (2010) | To analyse the effect of a personalised exercise programme on blood biochemistry values and other variables related to exercise in CKD patients | RCT: 2 groups, intervention vs. control: (n=45) vs. (n=49)Duration: 12 weeks | F:M=20:74Age: 73.2 (59–78) yearsInclusion: patients with CKD stage III-V and stable kidney function without treatment with renal replacement therapy | Exercise adapted to the preferences and possibilities of each patientFrequency: 3–5 times/weekDuration: 30min per sessionWithout direct supervision | Cholesterol levels were reduced in the intervention group (p<0.001). No significant changes in haematocrit, haemoglobin or blood glucose (p>0.05) |
| Koskamadis et al. (2011) | To analyse the effects of physical activity in CKD patients | RCT: 2 groups, intervention vs. control: (n=18) vs. (n=14)Duration: 24 weeks | F:M=13:19Age: 59.1 (31–83) yearsInclusion: patients with CKD stage IV-V without renal replacement therapy. The exclusion criteria were age <18years, pregnancy and functional or cardiovascular impairment which severely limited exercise capacity | Aerobic exercise (walking) of moderate intensity (RPE of 12–14)Frequency: 5 times/week. Duration: 30min per sessionWithout direct supervision | Exercise tolerance improved in the intervention group after the programme (increase in RPE during training) (p<0.001). Quality of life also improved (FACIT-Sp questionnaire) (p<0.05) and weight was reduced (p=0.007) |
In the 21 studies reviewed, a total of 927 patients (of whom 603 were male) were included. Four studies13–16 excluded women to avoid differences attributable to gender.
The main causes of kidney disease reported in the studies included are primary and secondary glomerulopathies (13.2%), diabetes mellitus (11.6%), nephroangiosclerosis (12.7%) and polycystic kidney disease (2.2%).
Nine14,15,17–23 of the studies included offer information on the comorbidity of the sample. The mean prevalence of the most common diseases was: 30.8% for diabetes mellitus, 18.5% for hypertension, 8.9% for dyslipidaemia, 5% for cardiovascular disease and 3.8% for peripheral vascular disease.
Exercise programmesThe frequency of the sessions was three times per week in 14 studies,13–17,19,20,23–29 with an approximate duration of 30min. Some included weekly increases in the exercise time up to 45–50min per session.17,19,20,24,25 Two studies programmed two weekly sessions and another two included five sessions.30,31 Only one programme carried out daily sessions.19 The remaining programmes did not specify the frequency of the sessions.21,22,32
The intensity of the exercise in most of the studies was mild-moderate. By measuring it using the maximal oxygen uptake calculated, this varied between 40 and 60%.15,17,20,24,25,30 Other interventions established the intensity of the exercise on the Rating of Perceived Exertion (RPE) scale at a mean of 11–15 points out of 20.21,22,28,29,33 One study applied the “talk test”.32
Ten studies reported having been supervised throughout the entire intervention by professionals.15,20,23–27,30,31 Nine articles included a first guided phase (1–8weeks) with a second part without direct supervision, although they maintained regular follow-up of the patients via phone calls or interviews.14,18,19,21,22,28 The remaining studies reported not having carried out regular follow-up of the patients during the intervention.13,17,29,32
Outcome measuresGlomerular filtration rate was analysed in 11 studies, using the CKD-EPI equation in five of them15,17,19,20,30 and the MDRD (Modification of Diet in Renal Disease) equation in six of them21–23,26,27,33. Proteinuria was studied in six trials: three of them measured it using the albumin to creatinine ratio13,14,21 and three measured 24-hour urinary albumin.16,26,30 Only one of the studies included a measure of patient survival, as well as the time up to starting dialysis.30
Several outcome measures were used to evaluate physical condition. Aerobic capacity was analysed in 13 studies using peak oxygen uptake (VO2 max.), defined as the maximum amount of oxygen that the body can absorb, transport and consume per unit time.14–20,22–26,28 In another programme, the MET (unit of measurement of the metabolic rate), which is the amount of energy consumed by an individual at rest, was analysed.21 Another of the studies included used the rating of perceived exertion.
Functional physical tests were implemented in six studies, using the six-minute walk test (6MWT) for this.17,19–21,29,31
Muscle strength was measured in seven studies, measured by the number of reps of bicep curls19,20 and hand grips21 for upper-limb strength, and by the number of reps of the sit-to-stand (STS) test15,19,20,29 for lower-limb strength.
In the analysis of body composition, anthropometric measurements such as body mass index (BMI), collected in ten trials,14,15,18–23,26,28 or waist circumference, analysed in five of the articles included15,22,23,26,28 were used.
Health-related quality of life was analysed in six studies. Three of them used the SF-36 questionnaire,14,20,25 two studies used the Kidney Disease Quality of Life (KDQOL-36)18,29 and another used the Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being (FACIT-Sp) Quality of Life Tool.33
Evaluation of the quality of the studiesDespite the fact that the studies of this systematic review only included controlled clinical trials with random allocation, only 13 described the way in which the sequence was generated, with a score of 3 points on the Jadad scale.13–15,18,20–22,24,25,28,29,31,32 Eight articles had 1 point.16,17,19,23,26,27,30,33 None of the studies are described as double-blind.
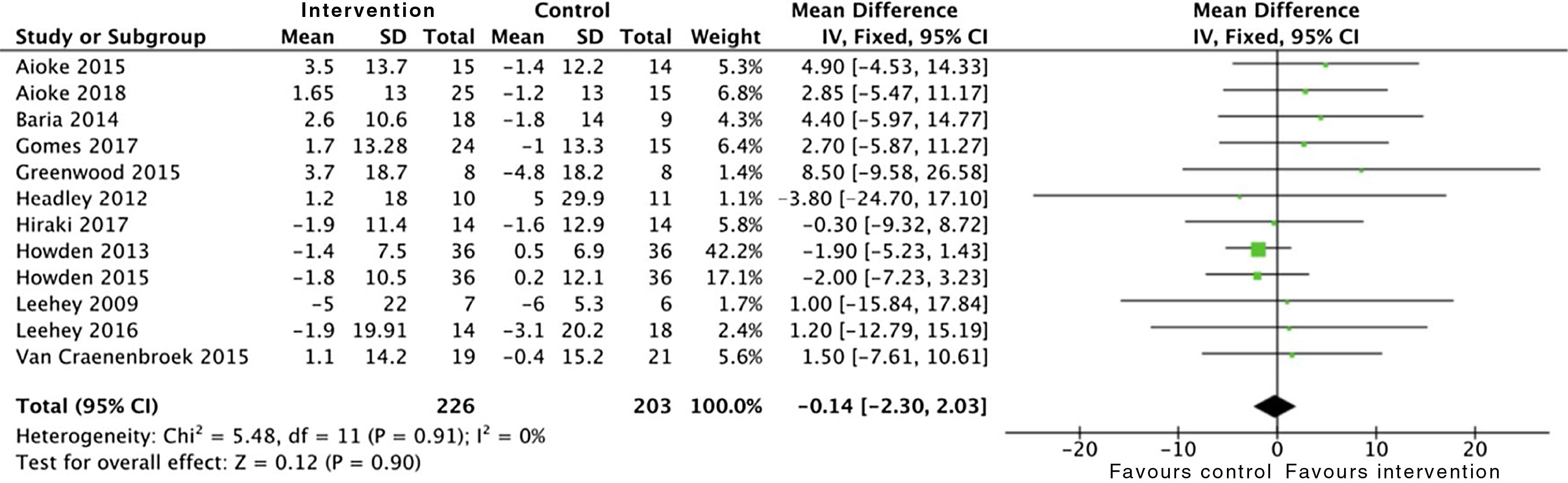
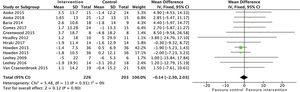
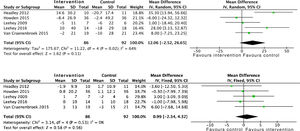
Effects on kidney functionThe analysis of grouped data from 429 patients demonstrated that physical activity does not have an impact on kidney function, with no differences observed in the estimated glomerular filtration rate between the intervention group and the control group at the end of the programme: SMD −0.1 (95% CI: −2.3 to 2.0; p=0.90) (Fig. 2).
Furthermore a long-term RCT showed that an aerobic exercise programme had positive, although insignificant, effects on the progression to end-stage CKD or the need to start RRT; OR 0.2 (95% CI: 0.01 to 4.9; p=0.32).30
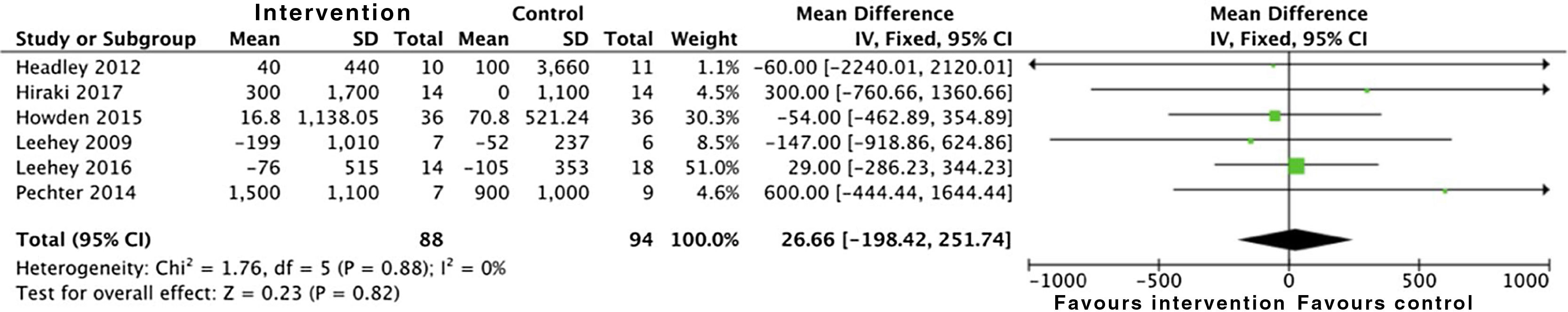
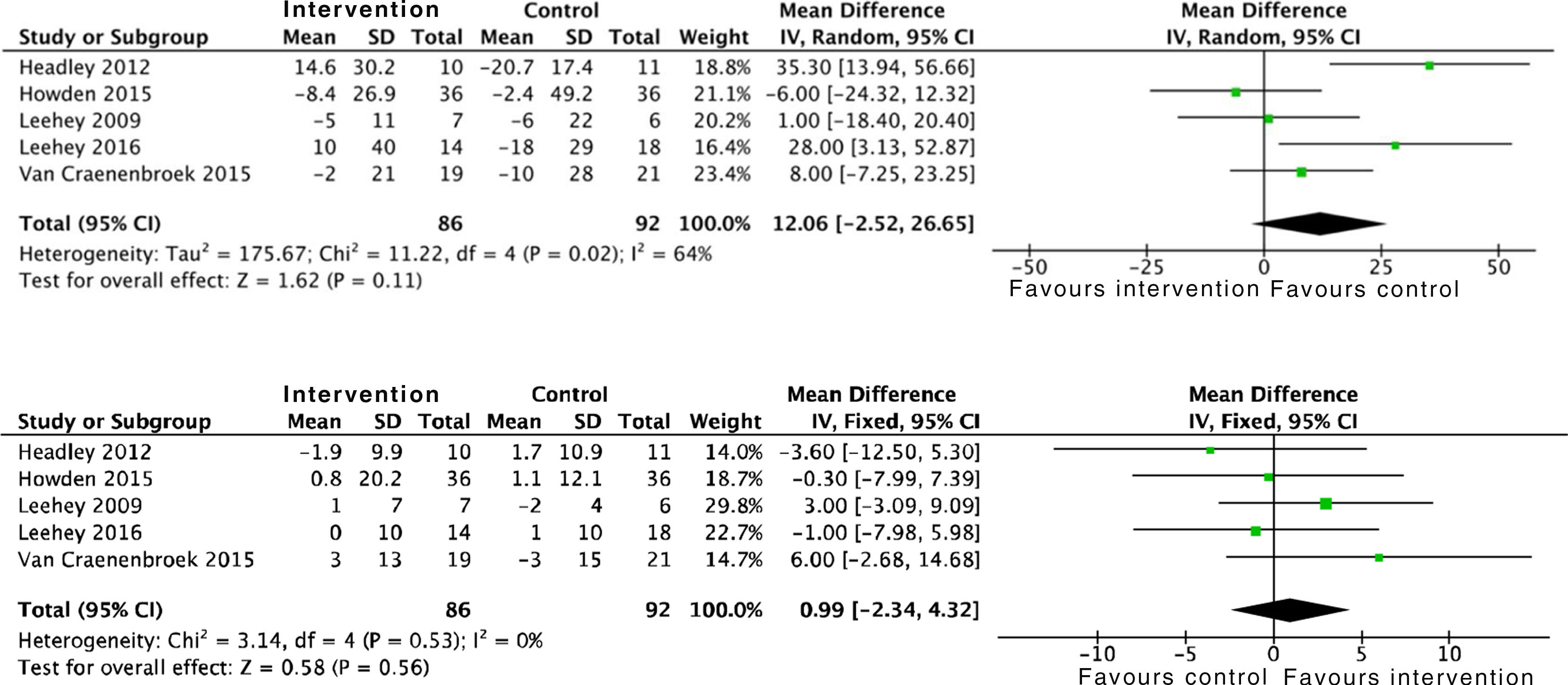
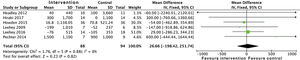
Proteinuria was not modified by physical activity either, by analysing 24h urine protein and the albumin to creatinine ratio. A total of six studies were included which took into account data from 182 patients: SMD 26.6 (95% CI: −198.5 to 251.7; p=0.82) (Fig. 3).
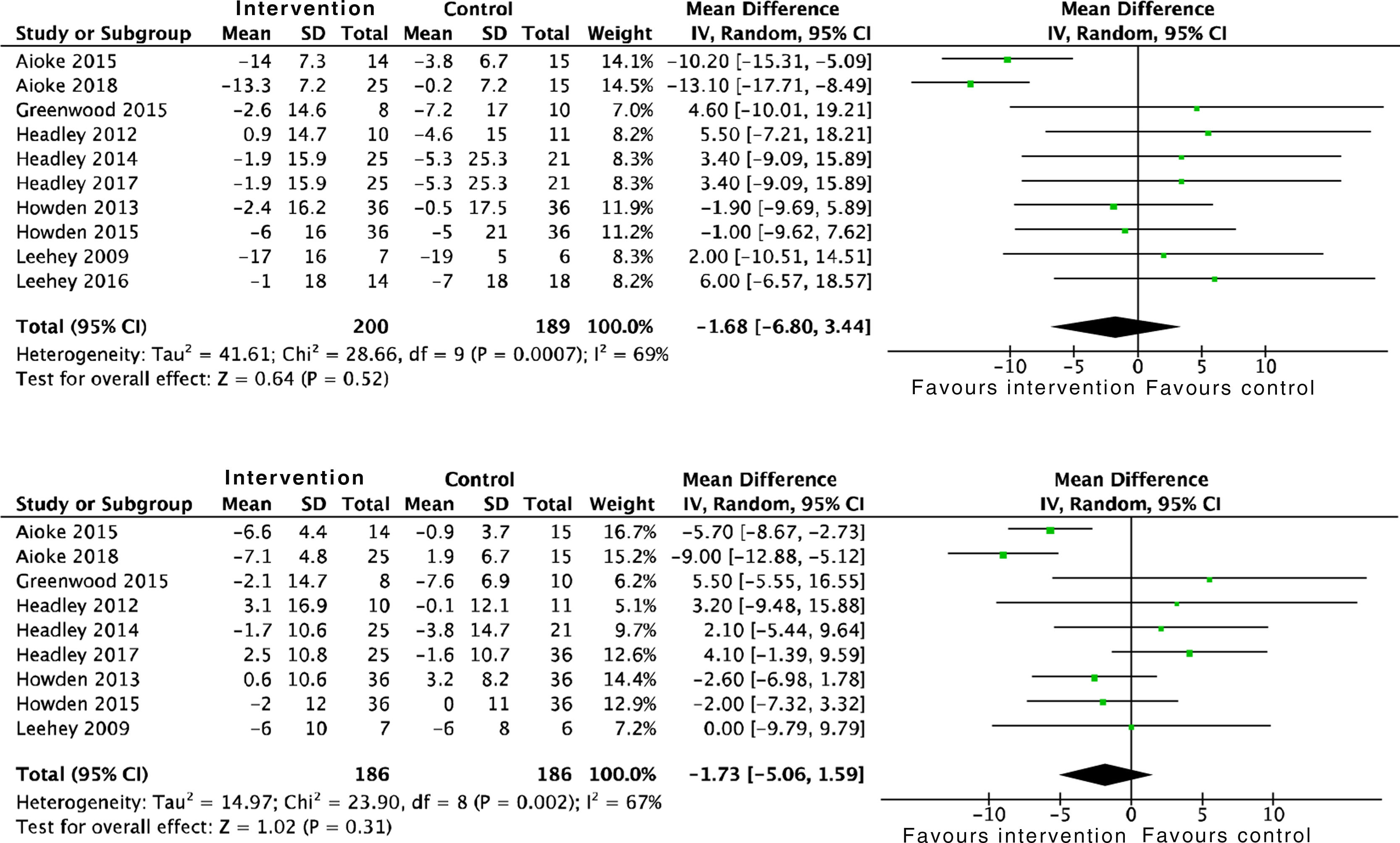
Cardiovascular and metabolic effectsThe effects of physical activity on systolic blood pressure (SBP): SMD −1.6 (95% CI: −6.8 to 3.4; p=0.52) and diastolic blood pressure (DBP) are conflicting: SMD −1.7 (95% CI: −5.0 to 1.5; p=0.31), according to data obtained from the analysis of 389 and 372 patients, respectively. Both meta-analyses showed positive, although insignificant, effects (Fig. 4). Only one study analysed left ventricular mass using echocardiography, with no differences found in the intervention group compared to the control group (p=0.98).22
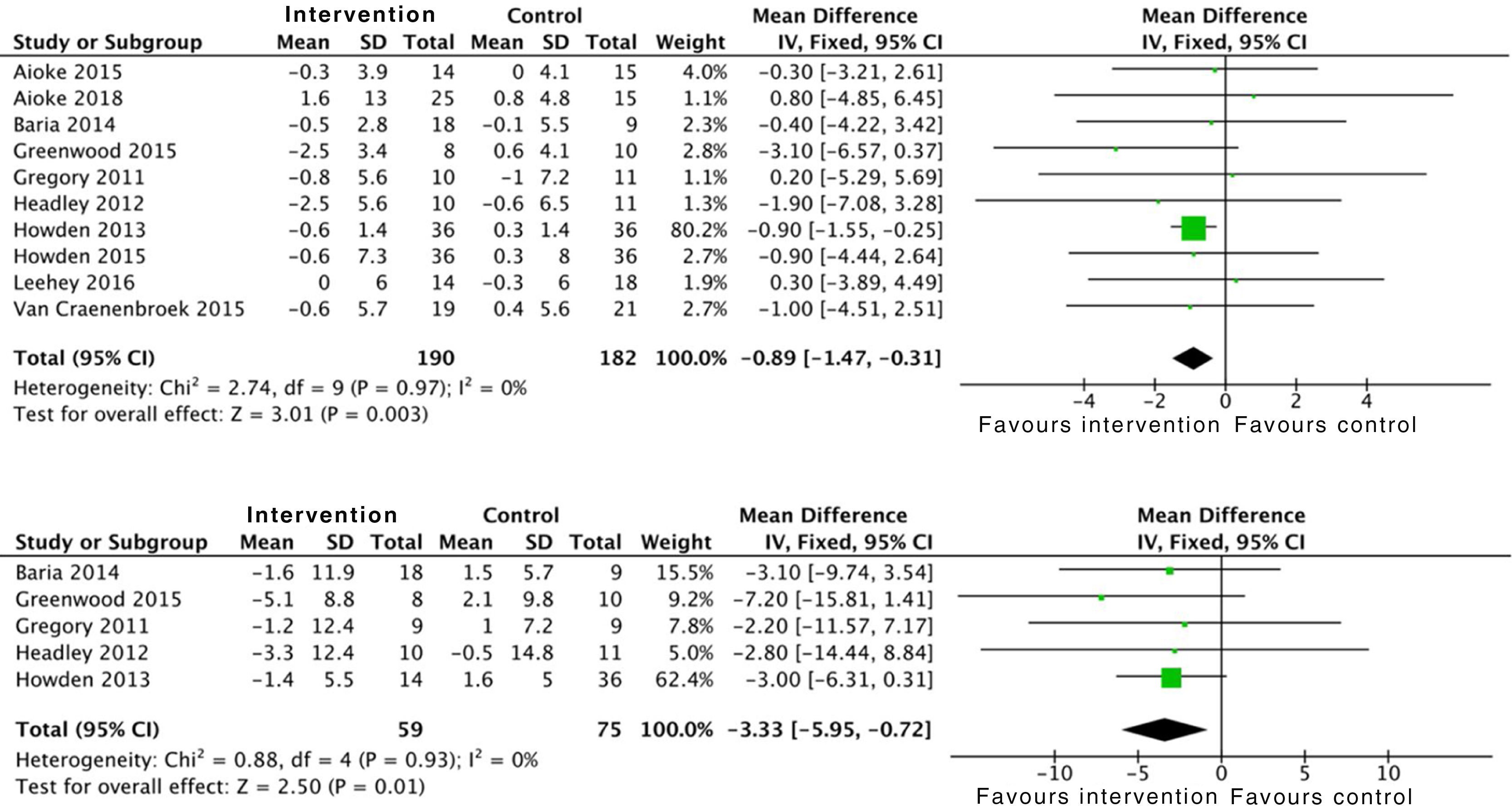
Some studies analysed the effect of exercise on haemoglobin, reflecting positive results on this with a high level of evidence. Grouped data from 168 patients were included: SMD 0.3 (95% CI: 0.1 to 0.5; p=0.003).16,19,32,33
There is conflicting evidence on the effect of physical activity on LDL cholesterol control: SMD 12.0 (95% CI: −2.5 to 26.6; p=0.11). We are not able to make recommendations regarding HDL cholesterol levels. Data obtained from the meta-analysis show positive effects, although these are not statistically significant: SMD 0.9 (95% CI: −2.3 to 4.3; p=0.56) (Fig. 5).
In three of the trials included, the effect of physical activity on glycated haemoglobin was analysed. The results obtained from the analysis of 115 patients did not show statistically significant differences between both groups: SMD 0.1 (95% CI: −0.56 to 0.57; p=0.98).14,16,21
Only two works analysed other factors related to cardiovascular risk in CKD patients, such as phosphorus, calcium, parathyroid hormone (PTH) and vitamin D. No statistically significant differences were found between the intervention group and the control group in either of them.17,33
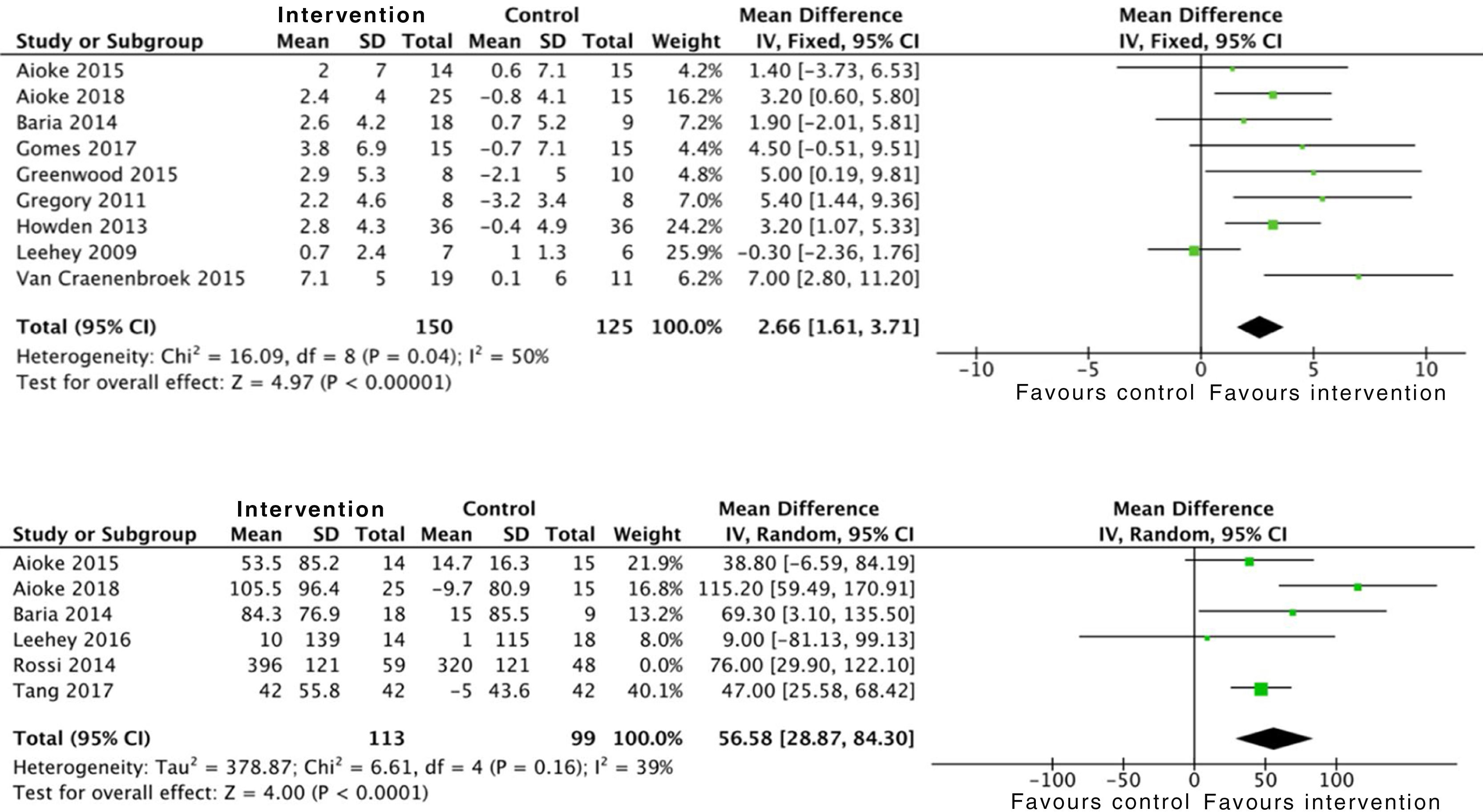
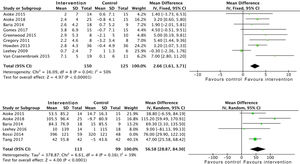
Effects on physical conditionAerobic exercise combined with resistance exercise in the short and medium-term has significant positive effects on physical condition, measured using peak oxygen uptake in the stress test, as demonstrated in the data obtained from 275 subjects: SMD 2.6 (95% CI: 1.6 to 3.7; p<0.001). Trials which used MET and the RPE showed similar results.21,33 In addition, the analysis of six studies which included 212 patients demonstrated an improvement in functional capacity measured by the 6MWT: SMD 56.5 (95% CI: 28.8 to 84.3; p<0.001) (Fig. 6).
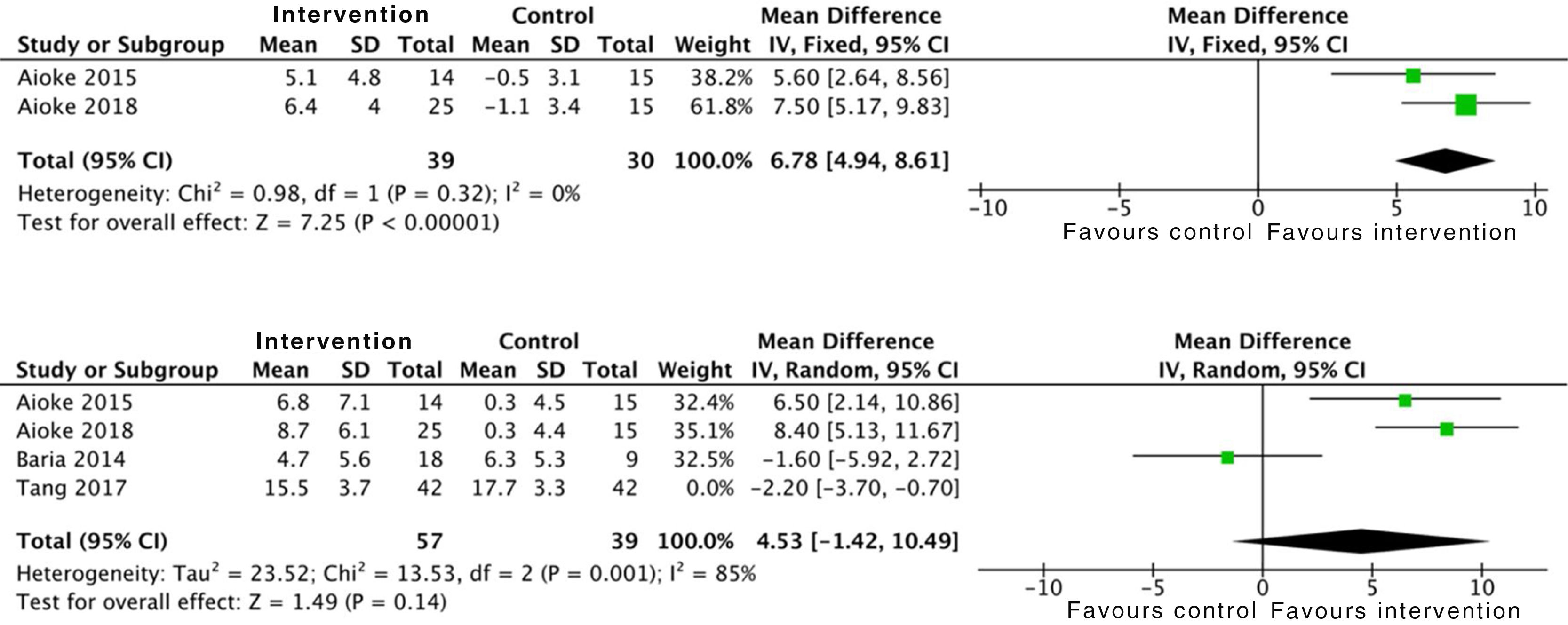
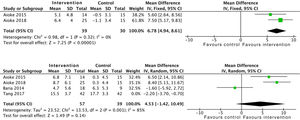
In relation to limb strength, physical activity improves upper limb strength, measured by the number of bicep curl reps: SMD 6.7 (95% CI: 4.9 to 8.6; p<0.001). Studies which measured upper limb strength using dynamometry offer insignificant positive results: SMD 2.1 (95% CI: −1.5 to 5.7; p=0.25).17,21,24 On the other hand, evidence is conflicting on lower limb strength. Data were collected from 96 patients who showed positive, although insignificant, effects: SMD 4.5 (95% CI: −1.4 to 10.4; p=0.14) (Fig. 7).
The effect of physical activity on anthropometric variables was also assessed in some studies. There is clear evidence on the positive effect of combined aerobic exercise and resistance exercises on BMI: SMD −0.8 (95% CI: −1.4 to −0.3; p=0.003). Exercise is also related to a reduction in waist circumference: SMD −3.3 (95% CI; −5.9 to −0.7; p=0.01) (Fig. 8).
Effects on quality of lifePhysical activity offers benefits in terms of health-related quality of life. Of the three studies that used the SF-36, the meta-analysis of two of them showed statistically significant positive effects in the physical section: SMD 6.66 (95% CI: 0.91 to 12.40; p=0.02).18,25 The third study presented the sum of each one of the questionnaire items independently, meaning that it was not possible to include it in the meta-analysis.31 The studies which used the KDQOL-36 questionnaire presented improvement when analysing the effects of the disease on quality of life: SMD 3.56 (95% CI: 0.49 to 6.63; p=0.02).19,29 Only one study used the FACIT-Sp scale, obtaining positive results.33
Effects on mortalityWhen analysing the effects on morality in patients with CKD, it was only possible to include a single study. This is a high-quality RCT (score of 3 points on the Jadad scale) with a follow-up of ten years that shows a positive, although insignificant, effect: OR 0.1 (95% CI: 0.01 to 2.8; p=0.1).30
DiscussionThe objective of this review was to describe the effect of an exercise programme on the progression of kidney disease and other factors associated with CKD. In previous studies, physical activity was not traditionally recommended for CKD patients due to the fact that the increase in sympathetic nerve activity during physical activity reduces renal blood flow, which could alter the intimate structures of the kidneys and affect their functioning.8,9 The review of the studies included in this meta-analysis shows that exercise is not related to changes in glomerular filtration rate. However, only nine of the reviewed studies analysed the impact of physical activity on the progression of kidney disease, three of them with low quality, which is why we should interpret these results with caution.15,17,19–22,28,30 Pechter et al.30 conducted the study with the longest follow-up of the review, including as a final study point the initiation of RRT or all-cause mortality. However, it is necessary to take into account that it is a study with a very small sample, with seven patients in the intervention group and nine in the control group. Over the ten years of follow-up of the water aerobic exercise intervention, they found that no one in the intervention group reached the end point of the study, while 55% of the control group had to undergo dialysis or passed away. Nevertheless, these differences were not statistically significant between both groups. Similarly, the review published by MacKinnon et al.34 concluded that a better physical condition and doing exercise were correlated with a lower risk of mortality, as well as a deterioration of kidney function, and a higher graft survival rate in transplanted patients. Other studies identified similar results on mortality in a population on haemodialysis.35
Proteinuria is also a fundamental risk factor in CKD progression. It is known that certain situations, such as intense exercise, can contribute to the onset or the increase of proteinuria.36,37 In the review carried out, doing moderate physical activity did not lead to a higher degree of proteinuria, which is why it could be prescribed safely in patients with CKD.13,14,16,21,26,30
The effects of physical activity on aerobic capacity are well known, and this has been seen in the articles included in this meta-analysis.16–20,22,23,28 This group of patients presents very little tolerance to exercise due, among other reasons, to the accumulation of the body's waste products, anaemia and muscle mass loss. All of this leads to lower levels of physical activity compared to the general population.38 Exercising is seen to provide a significant improvement in functional capacity and aerobic capacity, with our results coinciding with those of another meta-analysis recently published by Vanden-Wyngaert et al.39 Therefore, we can confirm that there is a high level of evidence for the improvements that an exercise programme brings to functional capacity, with results being observed from the first 12 weeks of intervention.14,15,19,20,29,31 Furthermore, the increase in haemoglobin levels with training, observed in the review that we have carried out, may contribute to this improvement, reducing the feeling of asthenia.14,16,21
CKD is a significant risk factor for cardiovascular morbidity and mortality. The increase in CKD in recent decades has been parallel to the increase in obesity, diabetes and metabolic syndrome. The already-commented on improvement in anaemia associated with exercise may provide these patients with an obvious benefit in terms of cardiovascular health. Furthermore, physical fitness could improve other aspects such as blood pressure.40,41 Better control of blood pressure is very important in these patients, as they have a high cardiovascular risk and hypertension is a recognised factor for kidney damage and CKD progression.42 In the studies of this meta-analysis, it is observed that doing aerobic exercise offers better control of SBP levels in the short and medium-term, although without reaching a significant difference.14,16,19–22,24–26,28 In terms of DBP, there is no evidence to make recommendations for this either.14,19–22,24–26,28
HDL and LDL levels were not modified with physical activity.14,16,18,21,26 It is important to remember that the lipid profile of CKD patients is different to that of dyslipidaemia in the general population, and it varies according to the severity of the kidney dysfunction, which makes the interpretation of the results gathered in the different studies of this review difficult. Dyslipidaemia in CKD is characterised by hypertriglyceridaemia, variable levels of LDL cholesterol and low levels of HDL cholesterol. In the initial stages we still found elevated levels of LDL cholesterol, but in the more advanced stages this parameter normalised or even reduced. The National Observatory of Atherosclerosis in Nephrology (NEFRONA project) observed a progressive decrease in total cholesterol, LDL cholesterol, HDL cholesterol and non-HDL cholesterol levels, proportional to the stage of the kidney disease.43
Glycated haemoglobin was not modified with physical activity according to the results obtained in this review. Certain works relate higher levels of glycated haemoglobin to a higher mortality rate in patients on haemodialysis. However, various factors such as anaemia and use of erythropoiesis-stimulating agents or iron supplements mean that the presence of CKD alters the relationship between blood glucose and haemoglobin, which makes their interpretation and their applicability in these patients difficult.44 Furthermore, hyperphosphataemia and secondary hyperparathyroidism have been linked to higher cardiovascular morbidity and mortality in CKD patients. Some works relate the practice of high- and low-intensity physical activity to changes in bone metabolism, along with a mild reduction in PTH.45 In this meta-analysis, no statistically significant differences were found, although the trials included were of low quality, which is why more studies are necessary in order to be able to make recommendations in this regard.17,33
In this review, we analysed anthropometric variables which were also related to cardiovascular disease, such as BMI and waist circumference. In the first case, an exercise intervention showed a significant reduction in BMI.14,15,18–23,26,28 With regard to waist circumference, patients had a lower circumference upon finishing the exercise programme.15,22,23,26,28 These results are important, as both variables have been reported as predictors of cardiovascular risk and CKD progression.46
As CKD progresses, complications also increase. Musculoskeletal disorders seem to be the main limiting factor of the ability to do exercises, and muscle breakdown is one of the strongest predictors of mortality in individuals with CKD.47 Based on the results provided by this meta-analysis, a training programme improved upper limb strength.19,20 However, more studies are needed to assess the results of exercise on lower limb strength: there were four clinical trials, one of them high-quality, in which positive, although insignificant, changes were found.15,19,20,29
The effects on quality of life are also of particular relevance. The symptoms of the disease itself limit physical activities, interfere with daily activities, intensify pain and its effect on everyday tasks and mean that the patient gives a worse assessment of their state of health.48 This review demonstrates that an aerobic exercise programme offers benefits to quality of life, improving the physical component, which has been described as the main factor that alters the quality of life of CKD patients who are not on dialysis or who have not received a transplant.14,18,25,26,29,31 A meta-analysis published by Zhao et al.49 reveals that physical activity can improve fatigue, anxiety and depression in patients with CKD, especially those on haemodialysis. Therefore, it is necessary to explore the appropriate type of physical activity for these types of patient, including it from the early stages to improve their physical condition and delay the progression of the disease.
The limitations of our study include the fact that the review and inclusion of the articles cited was carried out by one single investigator. However, the entire process and its results were subsequently supervised by the rest of the investigators. Other limitations that should be mentioned are the heterogeneity of the patients’ characteristics and the design of the clinical trials included. This meant that we encountered difficulties when analysing some data, given that the various articles used different measurement units, and in some of them the results or the exercise programme used were not detailed clearly. Despite this, the review was thorough, including all the articles which met the selection criteria and analysing all the parameters which were possible from the methodological point of view. Finally, only reports in Spanish and English were included; as a result, data from relevant studies published in other languages may be missing. Nevertheless, the articles in English and Spanish include the largest number of works and the publications with the greatest impact.
In conclusion, physical activity carried out routinely and at low-medium intensity offers significant benefits for CKD patients. It improves aerobic and functional capacity, impacting positively on perceived quality of life. It enables the control of blood pressure levels to be optimised, and improves certain analytical parameters such as haemoglobin. The most widely used routine is 30min per day, three to five times a week, with the intensity and frequency needing to be adapted to the patient's tolerability. The effects of exercise on kidney disease progression and the initiation of RRT are not clear, and the evidence regarding this is limited. However, it does not display negative effects on kidney function. Therefore, physical activity can be safely recommended in stable patients, and the results of this meta-analysis support the inclusion of exercise as a core element in the care of CKD patients.
FundingThis study has received no specific funding from public, private or non-profit organisations.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Villanego F, Naranjo J, Vigara LA, Cazorla JM, Montero ME, García T, et al. Impacto del ejercicio físico en pacientes con enfermedad renal crónica: revisión sistemática y metaanálisis. Nefrologia. 2020;40:237–252.