Patients with the dual burden of chronic kidney disease (CKD) and chronic congestive heart failure (HF) experience unacceptably high rates of symptom load, hospitalization, and mortality. Currently, concerted efforts to identify, prevent and treat HF in CKD patients are lacking at the institutional level, with emphasis still being placed on individual specialty views on this topic. The authors of this review paper endorse the need for a dedicated cardiorenal interdisciplinary team that includes nephrologists and renal nurses and jointly manages appropriate clinical interventions across the inpatient and outpatient settings. There is a critical need for guidelines and best clinical practice models from major cardiology and nephrology professional societies, as well as for research funding in both specialties to focus on the needs of future therapies for HF in CKD patients. The implementation of cross-specialty educational programs across all levels in cardiology and nephrology will help train future specialists and nurses who have the ability to diagnose, treat, and prevent HF in CKD patients in a precise, clinically effective, and cost-favorable manner.
Los pacientes con enfermedad renal crónica (ERC) que desarrollan insuficiencia cardíaca (IC) congestiva crónica presentan cifras inaceptablemente altas de síntomas, hospitalización y mortalidad. Actualmente, se echan en falta iniciativas institucionales dirigidas a identificar, prevenir y tratar la IC en los pacientes con ERC de manera multidisciplinar, prevaleciendo las actuaciones de las especialidades individuales. Los autores de este artículo de revisión respaldan la necesidad de crear equipos multidisciplinares cardiorrenales, en los que participen nefrólogos y enfermeras renales, que gestionen colaborativamente las intervenciones clínicas apropiadas en los entornos de pacientes con ERC e IC hospitalizados y ambulatorios. Es necesario y urgente que se elaboren guías y modelos de práctica clínica sobre la ERC con IC por parte de las sociedades profesionales de cardiología y nefrología, así como financiación para la investigación concertada entre ambas especialidades sobre la necesidad de futuros tratamientos para la IC en pacientes con ERC. La implementación de programas educativos cardiorrenales a todos los niveles en cardiología y nefrología ayudará a formar a los futuros especialistas y enfermeras para que tengan la capacidad de diagnosticar, tratar y prevenir la IC en pacientes con ERC de manera precisa, clínicamente efectiva y económicamente favorable.
Recently, the International Society of Nephrology adopted a proactive approach to defining the current state of kidney health through a multifaceted initiative aimed to close the gaps in care, research and policy. As part of this initiative, a group of experts identified several aspects of chronic kidney disease (CKD) that met criteria of unmet medical needs, among them the prevention and management of cardiovascular complications linked to CKD.1,2
Cardiovascular disease in patients with CKD is more frequent, more severe, and shows different manifestations compared with the non-CKD population, thus having a high economic and societal burden. Although the risk of conventional atherosclerotic events does increase when kidney function is reduced, most of the excess cardiovascular risk associated with CKD is due to non-atherosclerotic pathologies, such as left ventricular hypertrophy (LVH) with diastolic and systolic dysfunction, dysrhythmia, sudden cardiac death, cardiac valve disease, arterial calcification, and hemorrhagic stroke.3 Either ischemic or non-ischemic in origin cardiac diseases present in CKD patients are characterized by evolving to chronic congestive heart failure (HF) (i.e., cardiorenal syndrome type 4).4 Of note, the high death rates associated with all stages of CKD might reflect accelerated rates of both atherosclerosis and HF.5 On the other hand, individuals with heart disease and HF as a primary disorder can experience reduced kidney function as a secondary disorder, or both can coexist based on shared risk factors or systemic disorders.4 It is undeniable that the combination of CKD and HF is a growing health, economical and societal problem as the aging population leads to higher numbers of affected individuals. Therefore, a remarkable interest has been placed recently on hemodynamic, physicochemical, and biological processes through which the diseased kidney and/or the diseased heart interact to facilitate CKD and/or HF. In addition, common clinical scenarios call for recognition, knowledge, and skill in managing patients with CKD and HF.
In this article we will review in brief some aspects related to HF as a complication of CKD that make of it a true unresolved medical need and therefore deserve prompt and effective actions by the involved health professionals. In particular, we aimed to create awareness among the health professionals of the renal community (nephrologists and renal nurses) that the care of CKD patients with HF is currently one of the biggest challenges they face.
Burden and challengesThe epidemiological and economic magnitudeWhereas it is well known that between half and two thirds of patients with HF present values of estimated glomerular filtration rate (GFR) <60mL/min/1.73m2, with a greater prevalence in those with more severe symptoms and a stepwise increase in mortality risk with reduction of eGFR,6,7 the epidemiological figures of HF in patients initially identified as having CKD are not so well known.
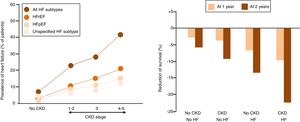
Prevalence of HF in CKDIn accordance to the US Renal Data System, in 2016, the prevalence of HF in CKD patients aged 65 and older was close to 26%, compared to 6% among patients without CKD.8 When patients were stratified in types of HF based on the presence of reduced or preserved left ventricular ejection fraction (HFrEF and HFpEF, respectively), or unspecified the prevalence of all types of HF were more common among those with CKD than among non-CKD patients and increased with greater severity of CKD stage. In this regard, an estimated 44% of patients with end-stage renal disease (ESRD) have HF (10% with HFpEF, 13% with HFrEF, and 21% with unspecified) (Fig. 1A).
(Panel A) Heart failure (HF) in patients with and without chronic kidney disease (CKD). (HFrEF, HF with reduced ejection fraction; HFpEF, HF with preserved EF). (Panel B) Adjusted survival of patients by CKD and HF status, 2015–2016.
Data on pre-transplant HF prevalence and prognosis are sparse, but the prevalence of left ventricular’LV’ systolic dysfunction/failure in patients carrying a renal transplant may be as high as 25%,9–11 despite that renal transplantation resulted in an increase in EF in more than 86% of recipients with HFrEF, and it was associated with an improvement in the New York Heart Association functional status in more than two-thirds of recipients.12
Incidence of de novo HF in CKDWhereas the worldwide incidence of de novo HF in the general population does not exceed of 1%,13 the incidence in known CKD is in the range of 17% to 21%.14 The cumulative probability to develop HF varies depending on the degree of CKD and the modality of renal replacement therapy (RRT), including transplantation. On the basis of Medicare billing claims data, the incidence of post-transplant de novo HF is ∼18% at 3 years.10
Of interest, in a study pooling participants without prevalent cardiovascular disease from 3 community-based cohorts from USA an estimated glomerular filtration rate (GFR) <60mL/min/1.73m2 (i.e., CKD stage 3a or higher) was found to be associated with an increased risk of HF that was similar in magnitude to ischemic heart disease and greater than stroke.15 However, the incidence of HF in renal transplant recipients has been reported to be considerably higher than that in the Framingham cohort, whereas the incidence of ischemic heart disease was not, suggesting that renal transplantation might correspond to a state of “accelerated HF”.16
Prognosis of HF in CKDThe presence of HF reduces the probability of survival among patients both with and without CKD, but to a greater extent among those with CKD (p-value for interaction <0.0001) (Fig. 1B).8 Over a two year period, patients with both CKD and HF had an adjusted survival probability of 77.8%, as compared to 90.2% for those with CKD alone, and 93.7% for those without HF or CKD.8
CKD was more strongly associated with mortality and had more prognostic discrimination in patients with HFrEF than in patients with HFpEF.17 The association of CKD with mortality in HFrEF is independent of age, functional class, duration of HF, hemoglobin, or diabetes mellitus.17
The presence of HF at the time of renal transplantation is associated with a higher risk of mortality, cardiovascular events, and graft failure.9,10,18 The ongoing burden of HF after renal transplantation is illustrated by the fact that HF accounts for 16% of all hospitalizations.19
The economic impact of HF in CKDThe data from the US Renal Data System on expenditures show the enhancer effect of HF on CKD.20 In the Medicare population aged 65 and older the per-person per-year costs in 2014 increased by 93% in patients with CKD and HF compared to patients with CKD alone. Data from Spain for the 2008–2010 period show that the direct and indirect health care costs related to HF are 58% higher in patients with estimated GFR values <60mL/min/1.73m2 than in patients with values ≥60mL/min/1.73m2 (14.868,2 euros per year vs 9.364,5 euros per year, respectively).21
The pathophysiological complexityThe mechanisms of organ injury and dysfunction in patients with CKD and HF are bidirectional with considerable overlap.
The chronically diseased kidney facilitates HF developmentIn CKD, hemodynamic risk factors for chronic HF include excessive afterload due to long-standing hypertension and arterial stiffness, and excessive preload due to salt and water retention.22,23 In addition, there are non-hemodynamic CKD-specific factors such as neurohormonal activation, excess of reactive oxygen species, pro-inflammatory cytokines and pro-fibrotic factors, impaired iron utilization, anemia, vitamin D deficiency, and retained uremic toxins that may also facilitate HF.22,23
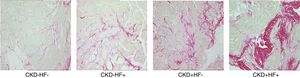
LVH is the principal initial cardiac structural perturbation in patients with CKD and the strongest independent predictor of cardiovascular mortality in these patients.23,24 Key histologic myocardial features underlying LVH include cardiomyocyte hypertrophy, thickening of intramyocardial arterioles, reduction of capillary density, and interstitial fibrosis (Fig. 2).25 Recently, it has been proposed that myocardial interstitial fibrosis, an almost constant finding in autopsy studies from patients at different stages of CKD, may be the major driver of cardiac dysfunction and failure in CKD patients.26 Initially, myocardial fibrosis enhances LV stiffness thus decreasing passive relaxation and impairing diastolic filling, and in advanced stages alters the alignment of cardiomyocytes thus impairing contractility and leading to systolic incompetence.27 It is plausible that pro-fibrotic mechanisms specific for CKD exist, among them an excess of fibroblast growth factor 23 that may serve as a biomarker and/or a therapeutic target of the cardiac involvement in CKD patients.28 There is strong evidence that abnormalities of LV structure and function, and the development of myocardial interstitial fibrosis are present in early stages of CKD, prompting the term CKD-associated cardiomyopathy instead of uremic cardiomyopathy.23,24
The failing heart further deteriorates renal function in CKDCumulative evidence supports that when chronic HF develops in the context of CKD both renal hypoperfusion due to low cardiac output and renal congestion due to elevation in cardiac pressures and preload act as major hemodynamic determinants of facilitating the progression of CKD.29 In addition, it is emerging the notion that the failing heart releases cardiokines, which can strongly impact various peripheral organs, as the kidney. For instance, cardiotrophin 1 (CT-1), a cytokine belonging to the interleukin 6 family produced and secreted in excess by the failing heart,30,31 has been shown to induce renal fibrosis and dysfunction through direct actions on the kidney.32 Of interest, an inverse association between plasm CT-1 and the estimated GFR has been reported in HF patients, and plasma CT-1 levels were significantly higher in patients with estimated GFR values <60mL/min/1.73m2 than patients with estimated GFR values ≥60mL/min/1.73m2.33
Although nearly a third of patients with stable chronic HF admitted for acute decompensated HF experience worsening renal function during the acute and post-hospitalization phases, its clinical impact in patients already presenting previous CKD has been scarcely evaluated and thus is currently unknown.34 Venous congestion instead of arterial underfilling, plus concomitant exacerbations of neurohormonal activation and inflammation are considered the main drivers of worsening renal function in the setting of acute decompensated HF.35
The diagnostic difficultiesThe 2016 European Society of Cardiology (ESC) HF Guidelines define HF as a clinical syndrome characterized by symptoms (e.g., dyspnea) and signs (e.g., elevated jugular venous pressure, pulmonary crackles and peripheral edema) of systemic congestion caused by a structural cardiac abnormality, resulting in a reduced cardiac output and/or elevated intracardiac pressures at rest or during stress.36 An elevated plasma concentration of natriuretic peptides (NPs) establishes an initial working diagnosis in a non-acute setting, identifying those who require further cardiac investigation (e.g., echocardiography).36
How diagnose HF-related congestion in CKD patients?Almost all patients with ESRD who do not receive RRT develop signs and symptoms consistent with HF, including dyspnea and edema due to inability of the severely diseased kidneys to excrete sodium and water. In addition, the severity of dyspnea in patients on intermittent hemodialysis changes with volume removal. Therefore, the definition of HF by the ESC has limitations when applied to patients with advanced CKD, namely those with ESRD undergoing hemodialysis. To address this gap, the Acute Dialysis Quality Initiative (ADQI) XI Workgroup proposed a functional classification of HF in ESRD, considering the timing of the assessment and periodicity of dialysis to classify HF symptoms in a patient on dialysis (Table 1).37
Proposed acute dialysis quality initiative classification for chronic heart failure in patients with end-stage renal disease according to the Acute Dialysis Quality Initiative XI Workgroup.
| Class | Echocardiographic heart disease | Presence of dyspnea | Relief of dyspnea with RRT or UF |
|---|---|---|---|
| 1 | Yes | Not | |
| 2R | Yes | On exercise | Yes |
| 2NR | Yes | On exercise | Not |
| 3R | Yes | With daily life activities | Yes |
| 3NR | Yes | With daily life activities | Not |
| 4R | Yes | At rest | Yes |
| 4NR | Yes | At rest | Not |
RRT, renal replacement therapy; UF, ultrafiltration.
As recently shown, only systemic congestion related to cardiac dysfunction and not overhydration per se is associated with higher mortality in ESRD patients undergoing hemodialysis.38 Therefore, the dissection of overhydration in cardiac and non-cardiac components in the individual ESRD patient is of primary clinical relevance since it provides prognostic information and might implicate different treatments. Although bioimpedance analysis is a popular tool in the assessment of overhydration in ESRD patients undergoing hemodialysis and the use of lung ultrasound for the detection of pulmonary congestion in HF is growing fastly in clinical practice, the two methodologies present limitations that challenge their ability to distinguish the underlying organ origin of overhydratation.
How diagnose a structural heart disease in CKD patients?On the other hand, structural heart disease, for instance LVH, is highly prevalent in patients with CKD, the prevalence increasing progressively with the loss of renal function and thus being present in more than 80% of patients with ESRD. Hence, the ADQI XI Workgroup proposed a new definition of structural cardiac disease in HF for patients with CKD, namely for patients with ESRD, according to the presence of at least one of eight echocardiographic alterations (Table 2).37 In all patients meeting one or more of those echocardiographic criteria, HF class is subsequently defined by the degree of dyspnea and by the response of congestive symptoms to diuretic therapy or dialysis and ultrafiltration (Table 1).37
The eight echocardiographic criteria suggestive of heart disease in patients with end-stage renal disease according to the Acute Dialysis Quality Initiative XI Workgroup.
| Parameters | Criteria | |||||||
|---|---|---|---|---|---|---|---|---|
| LV hypertrophy | LV dilatation | LV systolic dysfunction | Regional wall motion alteration | LV diastolic dysfunction | LA enlargement | RV systolic dysfunction | Valve disease | |
| LVMI, g/m2 | >130 men>110 women | |||||||
| LVMI, g/h2.7 | >50 men>47 women | |||||||
| LVEDV, mL/m2 | >86 | |||||||
| LVESV, mL/m2 | >37 | |||||||
| LVEF, % | ≤45 | |||||||
| Regional wall motility | Abnormal in >10% myocardium | |||||||
| Relaxation | Impaired | |||||||
| LA pressure | Elevated | |||||||
| E:A ratio | Grade II >0.8 and <2Grade III >2 | |||||||
| Average E:e′ ratio | Grade II 10–14Grade III >14 | |||||||
| PTRV, m/s | >2.8 | |||||||
| LAVI, mL/m2 | ≥34 | |||||||
| TAPSE, mm | <17 | |||||||
| Mitral valve stenosis or regurgitation | Moderate or severe | |||||||
| Aortic valve stenosis or regurgitation | Moderate or severe | |||||||
LV, left ventricle; LA, left atria; RV, right ventricle; LVMI, LV mass index; LVEDV, LV end-diastolic volume; LVESV, LV end-systolic volume; LVEF, LE ejection fraction; E, peak early diastolic velocity; A, peak late diastolic velocity; e′, average peak early diastolic mitral annular velocity at the septal and lateral acquisition sites; PTRV, peak tricuspid regurgitation velocity; LAVI, LA volume index; TAPSE, tricuspid annular plane systolic excursion.
However, as recently reported,39 echocardiographic criteria proposed by ADQI XI workgroup as a precondition for the clinical staging of HF (Table 2) are virtually omnipresent among CKD patients.11 By labeling a majority of CKD patients as having HF, application of ADQI criteria fails to specifically identify patients at high risk for future cardiac events. Therefore, it seems reasonable to assume the definition of HF proposed either by the ESC until the validity and utility of ADQI criteria have been shown in independent prospective studies.
How interpret NPs in CKD patients?CKD is one among the numerous causes of elevated NPs that may weaken their diagnostic utility in HF.40 Current data suggest that the cause of elevated NP concentrations in advanced CKD is multifactorial, representing in part a true counter-regulatory response from the heart to the kidney, and not only diminished passive renal clearance, as only 25% clearance of NPs is related to renal filtration.41 Indeed, two recent studies showed that patients with advanced CKD and HF with elevated brain natriuretic peptide (BNP) have very low neprilysin activity and postulated that BNP is a potent endogenous neprilysin inhibitor.42,43
To maintain optimal diagnostic performance, the cut-off concentrations for detecting HF may need to be raised when estimated GFR is <60mL/min/1.73m2.44 Due to the strong correlation between renal dysfunction and age, no additional adjustment seems necessary for N-terminal pro-brain natriuretic peptide (NT-proBNP) once using age-adjusted rule-in cut-offs. For BNP, the effect of renal dysfunction overall is smaller, and increasing the rule-out cut-off to 200pg/mL rather than 100pg/mL seems sufficient. Due to incomplete data, NP testing for HF should be discouraged in patients on dialysis.
The limitations to prevent and treatAlthough several independent risk factors for the development of HF have been identified among patients with CKD,45 few interventional studies have been performed attempted to verify their involvement. On the other hand, despite that patients with advanced CKD and concomitant HF are at particularly high risk of adverse outcomes the treatment of these patients presents currently several problems.
The paucity of data on HF prevention in CKD patientsHigh-quality data on prevention of HF in CKD are scarce. Intensive antihypertensive treatment aimed to a tight control of blood pressure has been shown to reduce incident HF in hypertensive patients with CKD.46,47 However, none of the 2 studies was specifically designed to investigate this effect. In patients with CKD and diabetes, treatment with the angiotensin II antagonist (AIIA) losartan,48 or with one sodium glucose cotransporter 2 inhibitor (SGLT2) reduce the risk of first hospitalizations for HF.49–54 However, as AIIAs and SGLT2s exert favorable effects on both blood pressure and glycemia, slow the progression of CKD, and display direct cardioprotective actions, the precise mechanism involved in HF prevention is actually unclear.
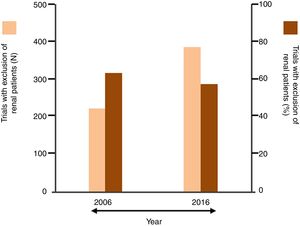
The undertreatment of CKD patients with disease-modifying HF therapiesIn the last 2 decades between half and two-thirds of cardiovascular trials excluded patients with renal dysfunction as assessed by variable degrees of increase in serum creatinine or decrease in estimated GFR.55–57 In particular, patients with stages 4 and 5 CKD were usually excluded from HF trials, often because of concern that the study drug might cause complications (Fig. 3).58 As a consequence, little evidence exists in support of treatment with beta-blockers, renin–angiotensin–aldosterone inhibitors, and sacubitril/valsartan in patients with advanced CKD. Accordingly, and in line with the caution expressed by contemporary HF guidelines regarding the treatment of patients with concomitant advanced CKD with HF drugs, this group of patients is mostly undertreated.
Summary of data from published systematic reviews reporting the percent of cardiovascular trials that excluded renal patients (thresholds applied for the exclusion: serum creatinine ≥1.5mg/dL or ≥2.3mg/dL or ≥3mg/dL, or estimated glomerular filtration rate <30mL/min/1.73m2).
The current evidence on treatment of patients with CKD and HFrEF has been reviewed in detail elsewhere.59,60 In particular, as regards the treatment of patients with advanced CKD and HFrEF, it seems that beta-blockers, angiotensin converting enzyme inhibitors, and sacubitril/valsartan may be used in these patients under surveillance to avoid hemodynamic deterioration, worsening renal function and hyperkalemia. On the contrary, mineralocorticoid receptor antagonists are generally contraindicated in patients with an estimated GFR of <30mL/min/1.73m2 as its use is associated with higher rates of all-cause mortality. As patients with advanced CKD and HF with high BNP levels exhibit very low neprilysin activity,42,43 the use of sacubitril/valsartan to treat these patents is questionable. Similar as for non-CKD patients with HFpEF no evidence-based treatment to reduce mortality outcomes exists for advanced CKD patients with HFpEF.61 To date, only weight reduction, exercise training, and diuretics have been shown to improve exercise tolerance and morbidity in HFpEF patients with or without advanced CKD.61 There are limited controlled data on the optimal pharmacotherapy of HF specific to renal transplant recipients. Management of HF in the context of renal transplant involves integrating available evidence-based therapies for HF in CKD (based on the degree of allograft function) as well as transplant-specific factors such as immunosuppressive agents.62
The dilemma of how treating congestion in CKD patientsManaging congestion is a critical point in the treatment of patients with CKD and HF, as it has been demonstrated that a close link exits between congestion and both worsening renal function,63,64 and HF events.65,66 Although diuretics are widely used to counteract sodium and water retention and reduce systemic congestion in HF, their use in patients with CKD requires particular considerations.67 Indeed, an excessive diuretic dose may lead to intravascular volume depletion and add pre-renal insults to established CKD. On the other hand, a diuretic-resistant state, defined as the inability to reduce congestion despite the use of high-dose intravenous loop diuretics, sequential nephron blockade and adequate fluid and sodium restriction may occur more frequently in patients with advanced CKD, than in patients with pre-existing normal kidney function. Therefore, the use of ultrafiltration may be considered.
The findings of a number of trials (Ultrafiltration Versus Intravenous Diuretics in Decompensated Heart Failure [UNLOAD], Continuous Ultrafiltration for Congestive Heart Failure [CUORE], Aquapheresis versus Intravenous Diuretics and Hospitalization for Heart Failure [AVOID-HF] and Cardiorenal Rescue Study in Acute Decompensated Heart Failure [CARRESS-HF]) demonstrate more effective decongestion with ultrafiltration than with pharmacological therapies.68–71 Whereas the UNLOAD, AVOID-HF and CUORE trials consistently showed greater reductions in HF events without greater increases in serum creatinine in the ultrafiltration compared to the diuretic arm, the CARRESS-HF trial showed no differences in 60-day outcomes between the ultrafiltration arm and the pharmacological arm, as well as a higher increase in serum creatinine in the former than in the later.71 Nevertheless, these data from the CARRESS-HF trial must be considered with awareness due to the inherent limitations of any per-protocol analysis as the one performed in this trial.
A meta-analysis on the effect of peritoneal dialysis to relief congestion in patients with CKD and refractory HF showed that in patients with CKD stages 1–4 the treatment was associated with reduction in body weight, improvement in cardiac function, maintenance of eGFR, and no changes in diuretic requirements.72 Thus, peritoneal dialysis can be an option to treat congestion in CKD stages 1–4 patients with refractory HF.
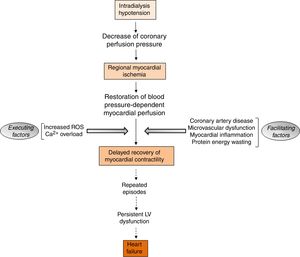
The aspects of RRT with potential detrimental cardiac impactIntradialytic hypotension complicates the management of volume status in ESRD patients undergoing hemodialysis. Intradialytic hypotension may be caused by aggressive ultrafiltration in response to interdialytic weight gain, and can lead to cardiac arrhythmias and myocardial stunning (Fig. 4).73 Myocardial stunning is characterized by delayed recovery of ischemia-induced regional myocardial contractile function after reperfusion despite the absence of irreversible damage and despite restoration of normal flow.74 Myocardial stunning over time is associated with permanent regional LV systolic dysfunction and HF,75 and an increased risk for cardiovascular death.76,77
There is evidence that in patients undergoing hemodialysis and in patients with a kidney transplant the vascular access may be a risk factor for HF as a high arteriovenous fistula flow is associated with LVH, LV dilatation and reduction of LV ejection fraction, as well as with development of pulmonary hypertension and right ventricular dysfunction.78–80 In addition, through its effect as a left-to-right extracardiac shunt, the arteriovenous fistula can increase cardiac workload substantially, and, in certain patients, leads to a high-output state and resultant HF over time.81
In renal transplantation mammalian targets of rapamycin (mTOR) inhibitors, are commonly used to offset the adverse effects of calcineurin inhibitor (CNI). A recent study showed that the supplementary administration of the mTOR inhibitor everolimus combined with a reduced-exposure CNI can reduce cardiac systolic function (i.e., reductions in LV fractional shortening) with no effect on diastolic function in the long term after renal transplantation.82 Whether this implies that the supplementary administration of everolymus for maintenance immunosuppression after renal transplantation should be avoided in recipients with HFrEF before transplantation deserves further study.
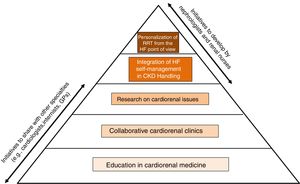
A call to actionClearly, extensive and robust information is lacking on all those aspects of HF specific to the population of CKD patients and discussed in the previous section. Therefore, the time has come for both nephrologists and renal nurses to develop initiatives that allow bridge knowledge and skills between nephrology, cardiology and other specialties in the field of CKD with HF (Fig. 5).
We need a new generation of cardiorenal nephrologists and renal nurses with an avant-garde approach to the screening, detection, diagnosis, prognosis, and management of cardiorenal patients, for instance those with CKD and HF. In this regard, the nephrological community should promote a union of cardiorenal medicine with cardiologists that in collaboration with internists and general practitioners should deal with cardiorenal patients.83
Achieving this goal undoubtedly begins with an innovative educational program to enhance the cardiac curriculums in nephrology training. A proposal for the framework and content of such an educational activity has been provided recently.84 This includes that nephrology fellows and renal nurses should spend a reasonable period of time in the HF unit of a cardiology department learning the diagnostic and therapeutic approach to the cardiac patient and the point of view of the paired specialty, providing answers to problems that currently seem insoluble in the renal clinical practice, including the management of HF in patients with CKD.85 Particular emphasis should be placed on the noninvasive cardiac imaging of the CKD patient, namely the patient with advanced CKD or with ESRD who is often remote from cardiovascular care.86 In this operative framework it is disappointing that in the “Training Guide for Nephrology Specialists” elaborate by the National Commission of the Specialty and approved by the Ministry of Education and Science of Spain in 2008 these aspects receive insufficient attention. Therefore, there is a need to update the Training Guide from the cardiorenal point of view.
In this context it is worth mentioning that among the 24 working groups belonging to the Spanish Society of Nephrology there is one properly dedicated to cardiorenal medicine that has been recently created, and that the guidelines of the Spanish Society of Nephrology on the kidney and cardiovascular diseases published 13 years ago are in the process of updating.87
Creation of cardiorenal clinics to care for patientsThe effectiveness of multidisciplinary HF management programs, both clinic-based and home-based, to improve outcomes (e.g., mortality and HF hospitalization) was established in the 2016 ESC HF guidelines.36 As stated in the Guidelines, key to the success of these programs is coordination of care along the continuum of HF and throughout the chain of care delivered by the various services within the health care system, including HF practitioners (primarily cardiologists, HF nurses and general practitioners) and other experts, including pharmacists, dieticians, physiotherapists, psychologists, palliative care providers and social workers. Unfortunately, nephrologists and renal nurses are not included in the list. The same is true when considering the standards developed by the Spanish Society of Cardiology to classify and establish the requirements for HF units in Spain.88
In this framework, the nephrological community must take up the challenge of integrating into the group of HF practitioners, especially when dealing with particular clinical scenarios and issues in which the interactions and synergies with other specialties may be necessary in the clinical decision-making process for CKD patients with HF (e.g., the application of concerted diagnostic strategies based on signs and symptoms, biomarkers, noninvasive imaging modalities, and invasive hemodynamic monitoring; the evidence-based use of goal-directed medical therapies for HF across the spectrum of GFR ranges; the choice of the optimal method to assess fluid status and to determine dry weight and appropriate decongestion strategies; the indication for implantable cardioverter-defibrillators; the usefulness of LV assist devices in cases of advanced HF; and the management of pulmonary hypertension in renal transplant candidates).
Research on uncovered cardiorenal issuesIn 2011 an international group of experts launched by the KDIGO and including nephrologists, cardiologists, neurologists, and representatives of other disciplines published an article reviewing the research needs of cardiovascular diseases, including HF, in CKD.3 In 2019 another international group of experts convened by the KDIGO published a review article on HF in CKD that included an outline of the prioritized research recommendations.4 It is surprising to see that most of the research proposals in the first article were still pending in the second article which obviously added new proposals.
It thus is time to create research platforms in basic, translational, preclinical, and clinical studies aimed both to gain insight into the mechanistic interactions between the chronically diseased kidney and the failing heart, and to improve future practice in CKD patients with HF in a precise, clinically effective, and cost-favorable manner. The recent introduction of clinically meaningful composite cardiorenal outcomes such as major adverse renal cardiovascular events and major adverse kidney events (Table 3)89 represents an important advance as allows the clinical consequences and the effects of different interventions to be defined more accurately.
Composite cardiorenal outcomes as novel target clinical endpoints in cardiorenal trials.
| Type of event | Composite MARCE | Composite MAKE |
|---|---|---|
| Cardiovascular | StrokeMyocardial infarctionHeart failure | |
| Renal | Need for RRT | Persistently impaired renal functionNew hemodialysis |
| General | Hospitalization for cardiac reasonsHospitalization for renal reasonsDeath | Death |
MARCE, major adverse renal cardiovascular events; MAKE, major adverse kidney events; RRT, renal replacement therapy.
Tertiary institutions, supported by the National Scientific Societies of medical specialties such as Cardiology, Internal Medicine and, of course, Nephrology should have a “task force” allocated to seek research opportunities in the field of CKD and HF.
Integration of HF self-management in CKD handlingHF patients experience symptoms of different intensities which impair their daily activities and reduce their quality-of-life. To cope with their clinical condition, many patients seek advice about self-management strategies when in contact with medical care providers. However, when analyzing some systematic reviews and meta-analysis published recently on self-management interventions proposed for patients with CKD stages 1–5 and patients on dialysis it is verified that the coexistence of cardiovascular conditions, in particular HF, is not considered at all to design the interventions or to evaluate the outcomes.90–92
The standards of self-management that patients with HF should expect have been published by the ESC Heart Failure Association (Table 4) and a number of practical recommendations to achieve them have been proposed.93 It would be desirable to apply these standards to CKD patients with HF. Renal nurses can play a key role in implementing self-management strategies for individuals with CKD and HF by encouraging collaborative, multidisciplinary working between these two disease conditions. This in turn will lead to enhanced communication, shared education programs and improve outcomes for patients.94
Recommended areas of interest, topics to be covered and goals to be achieved for self-management of patients with heart failure according to the European Society of Cardiology Heart Failure Association.
| Area | Topic | Goal |
|---|---|---|
| Education | Incorporate self-management | Optimize self-care |
| Diagnosis | Symptoms recognition | Anticipate or recognize deterioration |
| Remote patient monitoring | Anticipation of deterioration | |
| Prevention and treatment | Fluid and sodium management | Manage fluid status |
| Pharmacological treatment adherence | Use of compliance aids and proper motivation | |
| Dose titration | To ensure adequate titration to reach recommended target dose | |
| Nutrition and weight management | Keep a healthy body weight | |
| Immunization | Prevent infection-associated deterioration | |
| Lifestyle | Smoking and alcohol | Smoking cessation and prevention of excessive drinking |
| Physical activity | Maintain regular exercise | |
| Social life | Sexual advice | Acceptable sexual relationship |
| Depression | Recognize and treat | |
| Mobility and travel | To ensure the ability to move | |
The backdrop of high mortality, healthcare resource use, and poor quality of life in ESRD patients with advanced HF (e.g., stage D) suggests that patients with this dual burden would benefit from concurrent involvement with palliative care more than from RRT.95 In particular, the available evidence suggests that dialysis may not benefit older patients with ESRD and advanced HF.96
Thus far there are no randomized clinical trials to inform the benefits of peritoneal dialysis versus hemodialysis in patients with ESRD and HF. Strategies to maintain, where possible, residual renal function are desirable, as this can mitigate some of the significant hemodynamic and fluid shifts that occur with volume removal during dialysis. In this regard, it has been reported that patients starting on peritoneal dialysis show better initial outcomes and preservation of residual renal function in the first 2 years, compared with patients on hemodialysis but these differences normalize after 2 years.97 Compared to patients undergoing in-center hemodialysis, patients undergoing home dialysis have a markedly reduced risk of hospitalization for HF and cardiovascular mortality.98 In addition, short daily and nocturnal dialysis may be beneficial in patients with intradialytic hypotension because of the less abrupt fluid shifts associated with these approaches and reduced risk of hypotension and of hypotension-induced myocardial stunning.99 Additional studies are required to corroborate that dialysate cooling may protect from intradialytic hypotension-induced ischemic myocardial stunning.100
In this regard, it would be worth considering that renal nurses are trained and empowered to estimate intravascular volume status and fluid removal for potential prevention of intradialytic hypotension during a hemodialysis session using objective fluid or volume assessment measures other than systemic blood pressure or weight assessments before and after treatment (e.g., bioimpedance measurements and ultrasound of the inferior vena cava).101,102
While cases periodically arise where a fistula needs to be ligated with some urgency, such as severe HF, an increasingly persuasive case can be made for electively ligating fistulas that are not being used. The most common example of this is in stable renal transplant recipients, where the risks of ligation are outweighed by the potential risks of maintaining an unused fistula.103 As recommended by the Spanish clinical guidelines on vascular access for hemodialysis, surgically reducing the blood flow in large (usually upper arm) fistulas can be considered in patients with HF attributable to fistula-related volume overload.104 This means that access blood flow routinely should be measured and documented by trained nurses, namely in HF patients.
Concluding remarks and future directionsIn summary, HF in CKD appears to be a complex set of syndromes that represent a huge and urgent unmet medical need. Therefore, for the sake of CKD patients with HF, the health care system, and the society as a whole, it is now the right time to step forward from current nephrology to cardiorenal medicine. We make a call to enhance the curriculum in HF (and cardiovascular diseases, in general) to better equip both the nephrologists and renal nurses to handle the common, serious, and frequently fatal complications of CKD patients. A cardiorenal focused training will add value to nephrology subspecialties, potentially enhancing its attraction a significant subset of trainees.105 In addition, a nephrologist and a renal nurse with advanced cardiorenal training are valuable assets to any academic institution that seeks to develop interdisciplinary groups for the delivery of comprehensive care to complex patients, in addition to providing focused education to colleagues and trainees at various levels.
This means that it is mandatory learning from and working in a realistic manner with other disciplines with background in the implementation of HF medicine (i.e., cardiologists and internists), then disrupting the barriers among classical specialties and organizing the clinical practice around patients that are not exclusively renal or cardiac, but actually cardiorenal. In this regard, it is desirable, in large institutions, to develop fully combined programs resulting in board certification in both nephrology and cardiology. These institutions should also encourage nephrology faculties to develop career focus areas in cardiorenal medicine. The promise of true cardiorenal care in CKD patients with HF (and/or with other cardiovascular complications) should be one next goal of the Spanish Society of Nephrology and the Spanish Society of Renal Nursing for the forthcoming generations of renal doctors and nurses.
Conflict of interestNone.