The number of patients who start dialysis due to graft failure increases every day. The best dialysis modality for this type of patient is not well defined and most patients are referred to HD. The objective of our study is to evaluate the impact of the dialysis modality on morbidity and mortality in transplant patients who start dialysis after graft failure.
Material and methodsA multicentre retrospective observation and cohort study was performed to compare the evolution of patients who started dialysis after graft failure from January 2000 to December 2013. One group started on PD and the other on HD. The patients were followed until the change of dialysis technique, retransplantation or death. Anthropometric data, comorbidity, estimated glomerular filtration rate (eGFR) at start of dialysis, the presence of an optimal access for dialysis, the appearance of graft intolerance and retransplantation were analyzed. We studied the causes for the first 10 hospital admissions after starting dialysis. For the statistical analysis, the presence of competitive events that hindered the observation of the event of interest, death or hospital admission was analyzed.
Results175 patients were included, 86 in DP and 89 in HD. The patients who started PD were younger, had less comorbidity and started dialysis with lower eGFR than those on HD. The mean follow-up was 34 ± 33 months, with a median of 24 months (IQR 7–50 months), Patients on HD had longer follow-up than patients on PD (35 vs. 18 months, p = < 0.001). The mortality risk factors were age sHR 1.06 (95% CI: 1.03–1.106, p = 0.000), non-optimal use of access for dialysis sHR 3.00 (95% CI: 1.507–5.982, p = 0.028) and the dialysis modality sHR (PD/HD) 0.36 (95% CI: 0.148−0.890, p = 0.028). Patients on PD had a lower risk of hospital admission sHR [DP/HD] 0.52 (95% CI: 0.369−0.743, p = < 0.001) and less probability of developing graft intolerance HR 0.307 (95% CI 0.142−0.758, p = 0.009).
ConclusionsWith the limitations of a retrospective and non-randomized study, it is the first time nationwide that PD shows in terms of survival to be better than HD during the first year and a half after the kidney graft failure. The presence of a non-optimal access for dialysis was an independent and modifiable risk factor for mortality. Early referral of patients to advanced chronic kidney disease units is essential for the patient to choose the technique that best suits their circumstances and to prepare an optimal access for the start of dialysis.
El número de pacientes que inician diálisis por fracaso del injerto aumenta cada día. La modalidad de diálisis mejor para este tipo de pacientes no está bien definida y la mayoría de pacientes son derivados a HD. El objetivo de nuestro estudio es evaluar el impacto de la modalidad de diálisis sobre la morbilidad y la mortalidad en pacientes trasplantados que inician diálisis tras fracaso del injerto.
Material y métodosEstudio multicéntrico retrospectivo osbervacional y de cohortes que compara la evolución de pacientes que inician diálisis tras fracaso del injerto desde enero de año 2000 a Diciembre de 2013. Un grupo lo hace en DP y otro en HD. Se siguieron los pacientes hasta el cambio de técnica de diálisis, retrasplante o fallecimiento. Se analizaron datos antropométicos, comorbilidad, el filtrado glomerular (FG) con el que iniciaban diálisis, la presencia de un acceso óptimo para diálisis, la presencia de intolerancia al injerto y el retrasplante. Estudiamos el motivo de los 10 primeros ingresos hospitalarios tras el inicio de diálisis. Para el análisis estadístico se tuvo en cuenta la presencia de eventos competitivos que dificultaran la aparición del evento de interés muerte o ingreso hospitalario.
ResultadosSe incluyeron 175 pacientes. 86 en DP y 89 en HD. Los pacientes que iniciaron DP eran mas jóvenes, tenían menor comorbilidad y lo hacían con FG más bajos que los de HD. El seguimiento medio fue de 34 ± 33 meses, con una mediana de 24 meses (RIQ 7–50 meses), siendo mayor en los pacientes en HD que en los de DP (35 vs 18 meses, p = < 0,001). Los factores de riesgo que influyeron en la mortalidad fueron, la edad (sHR 1,06 (IC 95 %: 1,033−1,106, p = 0,000), el uso no óptimo del acceso (sHR 3,00 (IC 95 %: 1,507−5,982, p = 0,028) y el tipo de diálisis, la DP sHR[DP/HD] 0,36 (IC 95 %: 0,148−0,890, p = 0,028). Los pacientes en DP tenían menos riesgo de tener un ingreso hospitalario sHR[DP/HD] 0,52 (IC 95 %: 0,369−0,743, p = <0001) y menos probabilidad de desarrollar una intolerancia al injerto HR 0307 (IC 95 % 0,142−0,758, p = 0.009).
ConclusionesCon las limitaciones de un estudio retrospectivo y no randomizado, es la primera vez a nivel nacional que se demuestra que la DP en términos de supervivencia es mejor que la HD cuando fracasa el injerto durante el primer año y medio en diálisis. La presencia de un acceso no óptimo para diálisis es un factor de riesgo de mortalidad independiente y modificable. La remisión precoz de los pacientes a las unidades de enfermedad renal crónica avanzada (ERCA) es fundamental para que el paciente elija la técnica que más se adapte a sus circunstancias y preparar un acceso óptimo para el inicio de diálisis.
Nephrologists are faced every day with an increasing number of transplant patients in whom the kidney graft fails and must return to dialysis. In recent years, in Spain, between 2 and 4% of people who started dialysis did so due to kidney graft dysfunction. In absolute numbers, this figure represents about a thousand patients per year.1,2
Informing people with non-transplanted kidney failure about which modality of renal replacement therapy best suits their circumstances can be straightforward, but in those with kidney graft failure, this decision is complicated. The evidence base for that advice remains incomplete, as survival comparisons between dialysis modalities are fraught with methodological difficulties, in which a randomized trial is nearly impossible to perform.
There are few publications that evaluate the impact of the dialysis modality on the survival of the transplanted patient with a non-functioning graft who returns to dialysis. Most are local series with few participants in which similar results are reported in relation to survival between peritoneal dialysis (PD) and hemodialysis (HD).3–6 Currently, only one study demonstrates the superiority of the DP in terms of mortality compared to the HD during the first year after graft failure in July. None of these studies have taken into account the presence of competitive events for statistical analysis. By competitive event we understand that which makes it difficult or modifies the possibility of observing the event of interest.8,9 In such a way that, when we are studying death or the rate of hospital admissions in a certain dialysis technique, the abandonment of this by transplant or by transfer to another type of dialysis supposes a competitive event, since the patient ceases to be at risk of dying or having a hospital admission in the dialysis technique under study. In the presence of competitive events, the usual methods of time-to-event analysis such as the Kaplan–Meier survival curves are not suitable as they can overestimate the real event rate.10,11
It is a known fact that transplant patients who are on PD have a high rate of transfer to HD due to the rapid loss of residual renal function (RRF),4,12 therefore, the analysis, taking into account the competitive events, should be a priority when we want to study survival in a certain dialysis modality.
The aim of our study was to analyze the impact of dialysis modality (PD vs. HD) transplant patients returning to dialysis on mortality and morbidity (hospitalizations and death), taking into account the presence of competitive events.
Material and methodsPatient selection and study designThis is a multicenter, retrospective, observational and cohort study comparing the evolution of two groups of transplant patients who presented kidney graft failure and started dialysis from January 2000 to December 2013. A cohort started this procedure through DP and the other did it through HD. The patients were followed up until their death, change of dialysis technique, new transplant or end of the study (December 2013).
The PD cohort comes from transplant patients who presented graft dysfunction and started PD in hospitals belonging to the PD Levant Registry. This is a demographic, clinical and analytical database of people in PD in which 18 hospitals in the Valencian Community, Murcia and Albacete participate from 1990 to the present. From the registry data, we included all patients who started PD after graft failure from January 2000 to December 2013 and who were older than 18 years. We excluded those with a graft survival of less than three months.
The cohort of HD patients comes from those transplanted at the Dr. Peset University Hospital in Valencia and who, after transplantation, presented graft dysfunction and started HD in this center, in their hospitals of origin or in concerted HD centers dependent on the hospital from January 2000 to December 2013. In the years of follow-up of the study, at the Dr. Peset University Hospital, 4.8% of the patients with graft failure started PD and 95.2% did it in HD. We excluded those who started HD in hospitals or in non-hospital dialysis centers in which their medical history could not be accessed and those with a graft duration of less than three months.
Data collectAnthropometric variables, etiology of renal failure, cardiovascular risk factors, date of graft failure and initiation of dialysis, estimated glomerular filtration rate (eGFR) were analyzed using modification of diet in renal disease (MDRD) in mL/min/1.73 m2 and treatment with angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists (ACEIs/AIIRAs) at the time of dialysis initiation. We also analyze the beginning of the dialysis technique through a suboptimal access. It not considered optimal access start HD through a central venous catheter, temporary or permanent and DP, using the peritoneal catheter before 28 days required for maturation.13 We record the cause and date of death. We analyzed the data of the first ten hospital admissions after the start of dialysis, the date and the reason for admission. We reviewed the presence of graft intolerance and the need for embolization and/or transplantectomy. We document those patients who were put back on the waiting list and who agreed to a kidney transplant.
Follow-up was carried out from the time the patient began dialysis due to graft failure until he left it due to the end of the study (death or December 2013) or due to loss of follow-up (change of dialysis technique, transplantation or recovery of kidney function). To calculate the rate of hospital admissions, follow-up was carried out from the start of dialysis until leaving the study or until the date of the tenth admission. The exposure variables were PD and HD treatment and the outcome variables were death, hospital admission, graft intolerance and retransplantation. The objective of the study was to estimate these values and their association with the choice of technique (HD or PD).
Statistical methodThe results of the qualitative variables are expressed by absolute and relative frequency. Continuous variables are shown by the mean ± standard deviation or median and interquartile range (25th and 75th percentile), depending on whether or not they are normally distributed. The comparison of qualitative variables between the PD and HD groups was carried out using the X2 test. Quantitative variables were compared using the Student’s t test or the Mann-Whitney test, depending on whether the distribution was normal or not.
The raw survival of the patient was analyzed by performing Kaplan–Meier curves, comparing the results by means of the Breslow test and exposing the tables of individuals at risk. The graph includes the follow-up time until less than 10% of patients remain at risk.14
To determine the association of the dialysis modality (HD vs. PD) on survival and on hospital admissions (main objective), a competitive risk analysis was carried out, considering as such: the change of dialysis modality, the performance of a transplant and the recovery of renal function, since its appearance competed with that of death or admission.
Different models were carried out where those variables with a clinical sense about the study event and that met the proportional risk assumption were selected. The initial maximum model in each case included a number of variables, together with their possible interactions, that did not exceed 10% of the number of events. The final model was built behind a backwards or backwards strategy. The variables included in the final model for survival were: dialysis modality, age and suboptimal use of the access, and for hospitalization it was the type of dialysis. The criterion for evaluating confusion was a change of more than 10% in the sub Hazard Ratio (sHR) of the variable of interest (dialysis modality) when comparing a model adjusted for the possible confounder and one without adjustment. To control the selection bias dialysis technique performing a propensity index explored, but, due to the limited number of patients and homogeneity of groups, this did not converge properly. To determine the association of the dialysis modality on the appearance of graft intolerance and retransplantation (secondary objectives), a Cox analysis was performed. Due to the limited number of events, one could be included in the final model, in addition to the treatment modality, age and glomerular filtration rate (GFR) to the start of the dialysis.
Values of p < 0.05 were considered significant. The statistical package STATA® 13.1 (StataCorp, College Station, TX) was used for the analysis with competitive risks and the SPSS® 15.0 package (SPSS Inc., Chicago, USA) for the rest of the statistical analysis.
ResultsA total of 175 patients who started dialysis after graft failure were analyzed, 89 did so on HD and 86 on PD from January 2000 to December 2013. The mean follow-up of the entire series was 34 ± 33 months, with a median of 24 (IQR 7–50 months).
Baseline characteristics of the patientsTable 1 describes the baseline characteristics of the entire population. Compared with HD patients (n = 89), PD patients (n = 86) were younger and had less comorbidity. The eGFR at the beginning of the dialysis technique in the entire series was 13.1 ± 8.3 mL/min. The group of patients who started PD did so with a significantly lower eGFR than those who started HD (8.2 ± 4.2 vs. 17.3 ± 8.6 mL/min, p = 0.000). The follow-up time was shorter in the PD group, since there were more losses due to transplantation and discharges of the technique due to transfer to HD.
Clinical and analytical characteristics of the entire series, and divided by dialysis groups (PD vs. HD).
| DP | HD | p (DP vs. HD) | |
|---|---|---|---|
| n = 86 | n = 89 | ||
| Mean age, years (mean ± SD) | 45.8 ± 13.06 | 50.6 ± 13.17 | 0.018 |
| Year 2000−2004 | 42.6 ± 11.75 | 48.7 ± 16.4 | |
| Year 2005−2009 | 46.5 ± 13.71 | 47.4 ± 13.5 | |
| Year 2010−2013 | 48 ± 13.2 | 53.7 ± 10.8 | |
| Sex female, n (%) | 47 (55) | 57 (49) | 0.202 |
| Etiology of renal failure, n (%) | |||
| Glomerulonephritis | 28 (32.6) | 19 (21.3) | 0.300 |
| Not affiliated | 14 (16.3) | 19 (21.3) | |
| Interstitial | 16 (18.6) | 8 (9) | |
| Vascular | 4 (4.7) | 17 (19.1) | |
| Poliquistosis | 5 (5.8) | 14 (15.7) | |
| Others | 9 (10.5) | 2 (2.2) | |
| Systemic | 3 (3.5) | 7 (7.9) | |
| Diabetes | 7 (8.1) | 3 (3.4) | |
| Diabetes mellitus, n (%) | 11 (13) | 17 (8) | 0.198 |
| Dialysis mode | |||
| DPCA/DPA (%) | 76/24 | – | |
| HF-HD (%) | – | 100 | |
| Cardiovascular comorbidity, n (%) | 24 (28) | 56 (63) | <0.001 |
| Charlson index corrected for age* | 3.4 ± 1.5 | 4 ± 1.3 | 0.005 |
| Dyslipidemia, n (%) | 40 (47) | 51 (60) | 0.062 |
| ACEI/AIIRA treatment, n (%) | 44 (55) | 41 (62) | 0.242 |
| EGFR at the start of dialysis (mL/min) | 8.2 ± 4.2 | 17.3 ± 8.6 | <0.001 |
| Non-optimal use of access for dialysis, n (%) | 27 (34.6) | 19 (22.4) | 0.059 |
| Follow-up (months); median (IQR) | 18 (6−34) | 35 (10−70) | <0.001 |
| Loss to follow-up; n (%) | <0.001 | ||
| T rasplante | 16 (18.6) | 11 (12.4) | |
| Dialysis technique change | 26 (31.4) | 1 (1.1) | |
| Peritonitis (n) | 8 | – | |
| Ultrafiltration loss (n) | 8 | – | |
| infradialysis (n) | 4 | – | |
| Technical complications (n) | 6 | – | |
| Will of the patient (n) | 0 | 1 | |
| Recovery of kidney function | 1 (1.1) | 0 (0) | |
| Death | 10 (11.6) | 28 (31.5) | |
| Assets as of December 2013, n (%) | 33 (38.4) | 49 (55) | 0.034 |
SD: standard deviation; EGFR: estimated glomerular filtration rate; IQR: interquartile range; CAPD: continuous ambulatory peritoneal dialysis; APD: automated peritoneal dialysis; HF-HD: high flow hemodialysis.
Throughout the entire follow-up, there were 38 deaths in 175 people (22% of patients), 10 in the PD group and 28 in the HD group. 42% were cardiovascular, 15.8% infectious, 7.9% neoplastic, another 15.8% unknown, and 18.4% miscellaneous, with no differences between the two dialysis modalities. Patient survival throughout the series was 93% at 1 year and 70% at 5 years.
During the entire follow-up, a total of 439 admissions were analyzed in 129 patients (74%). The crude rate of hospital admissions for the global series was 0.89 admissions per year at risk (one every 13 months).
The most frequent cause of admission was cardiovascular with 121 (28%) followed by infectious with 96 (22.2%). Graft intolerance was the cause of 62 admissions (14.3%) and complications of the dialysis technique caused 59 (13.6%), the remaining 93 (21.5%) being due to various pathologies, the two most common were gastrointestinal bleeding and parathyroidectomy.
During the entire follow-up, 44 patients (25.1%) had some episode of graft intolerance, 35 of them (79%) required graft embolization and five (11%) a transplantectomy. The mean post-dialysis time to the onset of intolerance was 10 ± 9 months, with a median of 8 (IQR 5–13 months).
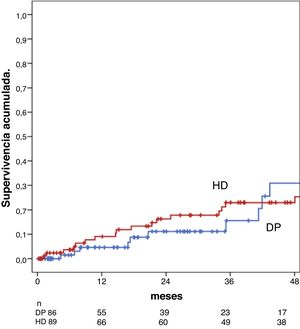
Association between dialysis modality and survivalThere were no differences in survival between the PD group and the HD group at one year or at five years: 95% in PD vs. 92% in HD at one year and 69% at five years in both groups, Breslow test, p = 0.30 (Fig. 1).
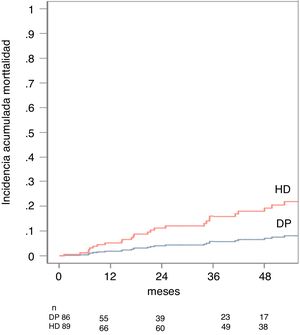
In the univariate analysis, mortality was related to the dialysis modality (sHR[DP/HD] 0.37 95% CI: 0.175 to 0.774, p = 0.008), age (sHR 1.05 95% CI: 0, 06 to 1.09, p = 0.000), a higher Charlson index (sHR 1.59 95% CI: 1.28–1.98, p = 0.000) and the presence of vascular comorbidity (sHR 2.78 CI 95 %: 1.41–5.50, p = 0.003. The predictive model established that the variables that best predicted patient mortality in our series were age with a sHR 1.06 (95% CI: 1.033–1.106, p = 0.000), the non-optimal use of access with an sHR 3.00 (95% CI: 1.507–5.982, p = 0.028) and the type of dialysis, in this case the PD obtained a sHR[PD/HD] 0, 36 (95% CI: 0.148 to 0.890, p = 0.028) (Table 2). The cumulative incidence of death adjusted by the competitive risk model at 1 year was 3% for PD and 11% for HD and at five 8% for PD and 21% for HD, p = 0.028 (Fig. 2).
Analysis by competitive risks, according to the modality of dialysis PD vs. HD (sHR). Ref 1 = HD.
| Variables | S HR | CI (95%) | p |
|---|---|---|---|
| Mortality | 0.36 | 0.148−0.894 | 0.02 |
| Hospitalization | 0.52 | 0.369−0.743 | <0.001 |
Mortality adjusted for: age, vascular comorbidity, suboptimal use of access. Hospital admission adjusted for: age, vascular comorbidity, glomerular filtration at the start of dialysis, and suboptimal use of access. SHR: sub Hazard Ratio.
The hospitalization rate was significantly lower for the PD group, 0.68 admission/patient/year (one admission every 17 months) than for the HD group, 1.01 admission/patient/year (one admission every 12 months), p = 0.0011. We did not find significant differences in the causes of admission between the two dialysis groups (PD and HD) (Table 3).
Description of all causes of admission in the global series and comparative analysis between both dialysis groups (p = 0.257).
| Cause entry | Everyone | DP | HD |
|---|---|---|---|
| n = 431 | n = 117 | n = 314 | |
| Cardiovascular, n (%) | 120 (28) | 40 (34.1) | 80 (25.4) |
| Infectious, n (%) | 98 (22.2) | 22 (18.8) | 76 (24.2) |
| Graft intolerance, n (%) | 63 (14.3) | 15 (12.8) | 48 (15.2) |
| Related to the dialysis technique, n (%) | 58 (13.6) | 15 (12.8) | 43 (13.6) |
| Others, n (%) | 92 (21.5) | 25 (21.3) | 67 (21.3) |
PD: peritoneal dialysis; HD: hemodialysis.
In the multivariate analysis, the initial model included age, suboptimal access use, vascular comorbidity, Charlson index, DM, and dialysis modality. The final model established that the variable that best predicted the probability of hospital admission of the patient in our series was the dialysis modality, which, in the case of PD, presented a sHR[PD/HD] 0.52 (95% CI: 0.369 a 0.743, p = < 0.001) (Table 2 ).
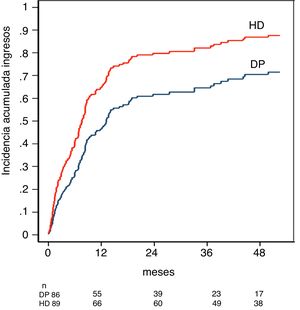
The cumulative incidence of hospital admission adjusted by the competitive risk model was 47% for PD and 63% for HD in the first year, and 71% for PD and 88% for HD at five years, p < 0.001 (Fig. 3).
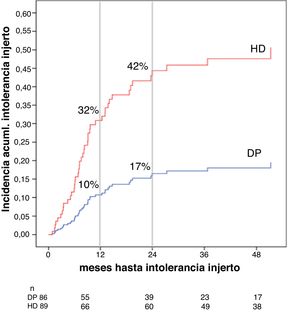
Association between the dialysis modality and the risk of graft intolerance syndromeThe cumulative incidence of graft intolerance was 32% at 12 months and 42% at 24 months for HD and 10% at 12 months and 17% at 24 months for PD (Fig. 4). Patients who developed graft intolerance had a higher eGFR at the start of dialysis (16 ± 9 vs. 12 ± 8 mL/min/1.72 m2, p = 0.016) and a higher percentage were on HD (32.8 vs. 11.6%, p = < 0.001), with no significant differenes regarding the time of presentation, median of seven months (IQR 5–12 months) in HD versus 12 months (IQR 6–20 months) in PD, p = 0.257. In the multivariate analysis, after adjusting for age and eGFR at the start of dialysis, PD was associated, less frequently, with the appearance of a graft intolerance syndrome, HR 0.307 (95% CI 0.142 to 0.758, p = 0.009) (Table 4).
Cox analysis for the study of risk factors for developing graft intolerance.
| Variable | HR | CI (95%) | p |
|---|---|---|---|
| PD dialysis mode (ref 1 = HD) | 0.307 | (0.142−0.758) | 0.009 |
| Age (year) | 0.994 | (0.962−1.009) | 0.22 |
| GFR at the start of dialysis | 0.998 | (0.962−1.035) | 0.89 |
PD: peritoneal dialysis; HD: hemodialysis; HR: Hazard Ratio; DF: glomerular filtration.
Of the entire series of patients, 88 (50.2%) were placed on the waiting list for a new transplant. Of these, 41 finished the follow-up without undergoing this procedure, 17 changed their technique before transplantation and 27 (15.4%) were transplanted while in the technique in which they started dialysis, three subjects died while being active on the waiting list. The mean time to retransplantation was 26 ± 24 months with a median of 22 (IQR 4–35 months). The patients who underwent the procedure were younger (39 ± 11 vs. 50 ± 13, years, p = 0.000) and a higher percentage were women (63 vs. 46%, p = 0.008) compared to those who did not undergo transplantation. In the multivariate analysis, only age turned out to be a factor associated with the probability of receiving a kidney transplant, in such a way that older people had a lower probability of receiving a transplant (HR 0.969, 95% CI 0.941 to 0.997, p = 0.032).
DiscussionTo our knowledge, this is the first study at the national level in which PD has been more advantageous in terms of morbidity and mortality than HD after renal graft failure, taking into account competitive events. In the literature, we find numerous publications that study the morbidity and mortality of transplanted patients who return to dialysis and compare it with those who have not been transplanted,12,15–19 but only five publications relate the impact of the dialysis modality on said morbidity and mortality.3–7 Our results have great clinical relevance, since we should make you reconsider the current trend of not including PD patients after graft failure, as the vast majority of those who return to dialysis do with HD.20
In our study, the patients who were included in PD after graft failure were significantly younger and with less cardiovascular morbidity than those who were admitted to HD, a fact that was consistent with most of the published series.3,6,7 This characteristic represents an important bias that can be partially corrected through statistical analysis adjusted to the clinical and demographic variables available.4,5 However, according to some authors,21 there are characteristics such as self-care capacity, responsibility towards dialysis treatment and compliance with it, which are not included in most studies and are therefore not available at the time of comparative analysis of dialysis groups. These characteristics, considered as “functional” are an important statistical confounder, in such a way that patients who had a greater degree of commitment to their disease, greater capacity for self-care and who enjoyed a better state of health, tend to prefer a technique home dialysis such as PD. On the contrary, individuals who are less disciplined, less compliant and therefore, with a potential for a worse prognosis, would choose HD, thus worsening the overall survival of this group.
In studies with large number of patients, the use of sophisticated statistical tools such as propensity test allows two comparable groups and partially eliminate selection bias technique.7 In our work, the possibility of performing a propensity test was explored, but it did not converge adequately, due to the limited number of patients, so its application was not possible. Consequently, one of the fundamental limitations is the presence of a selection bias that may justify, despite the statistical adjustment, that the group of people on PD present better survival than those included on HD after graft failure.
Another notable difference in our series was that HD patients started dialysis with higher eGFRs than those on PD. As was the case in the study by Perl et al.,7 we consider that this phenomenon could be related to the fact that the subject who begins HD does so more often due to episodes of heart failure derived from a worse clinical state and needs dialysis with higher eGFRs than those who do so on PD. The existence of a functioning internal arteriovenous fistula (iAF) or the ease of placing a central venous catheter and its immediate use could also favor this earlier onset. On the contrary, for those patients who wish to start PD, the implantation of a peritoneal catheter must be scheduled and a minimum time of maturation must be ensured, facts that could delay the start of dialysis for weeks or even months.
Another fact to highlight is that the percentage of diabetic people who start PD is only 13%. This is probably due to the low inclusion of diabetic subjects in PD in the hospitals of the Levante Registry, since, if we analyze the incorporation of patients in PD de novo (not transplanted), we also see that the percentage of diabetics is lower than that reported nationwide (14.9 vs. 25%).1,2
The mean follow-up of the entire series was 24 months, as in previous studies,7,18,22 being shorter in the PD group due to the need to transfer to HD due to the inability to obtain an adequate dose of dialysis. According to some authors, the cause of this insufficient dose could be related to the rapid loss of RRF in transplanted grafts, especially if it is decided to suspend immunosuppression after failure.4,23
In our series, the survival of transplanted patients after the start of dialysis was 93% in the first year and 70% at five years, data similar to those reported in most of the published studies.19,22
The main causes of death in our study were cardiovascular and infectious. These findings confirm that our results are comparable to those described in previous studies, both recent24 and old25 and show that the causes of mortality in these patients have not changed significantly in the last 15 years, indicating which are still the main causes. Challenges in the field of morbidity and mortality in transplanted patients who start dialysis.
The independent risk factors that increased mortality in our analysis were age, described in most of the published series,17,19,24,25 the HD modality with respect to PD, and the initiation of dialysis through non-optimal access.
In our research, PD has been associated with lower mortality compared to HD. In the literature, there are publications in both directions, in the majority, mortality is similar in both dialysis techniques when the graft fails3–6 while in others, such as the study by Perl et al.,7 mortality varies according to time on dialysis. In this work, the authors describe that, after the start of dialysis when the graft fails, PD is superior in terms of survival up to the first year, equals HD between the first and second year and, beyond two years, PD would have higher mortality than HD. According to these data, the better survival of the PD group in our series could be explained by the median survival in this group with the technique. Furthermore, based on these findings, one could speculate on the advisability of not delaying the transition to HD after a year and a half so as not to worsen patient survival.
Non-optimal access at the beginning of dialysis, both for HD and PD, behaves in our series, as in24 others, as an independent risk factor for death, a relevant fact, since it is a factor of modifiable risk and depends directly on the coordination between the transplant nephrologist and the dialysis nephrologist. Although in our study, the percentage of patients who started HD through a central venous catheter is lower than that of others,26,27 it is known that initiating HD through the central venous catheter increases the risk of sepsis and favors underdialysis. In PD, the use of a catheter without the appropriate maturation period increases the incidence of peritoneal fluid leaks, catheter exit site infections and, consequently, peritonitis, all of this limiting the possibility of achieving ultrafiltration targets (UF) and dialysis dose (Kt/V).13
With all this, given the irreversible deterioration of the graft function, the referral of the patient to pre-dialysis consultations should be done early to guarantee the creation of an AVF or the implantation of a PD catheter. In this way, we would be ensuring a mature access at the time when the patient must start dialysis and we would avoid a large number of complications. Coordination between the transplant unit and the different areas in charge of creating dialysis access is essential at this time, as Oppenheimer et al.28
The overall rate of hospital admissions in our series was similar to that reported in other cohorts of patients.22 The main reason for entering the study, like other publications,29 was the cardiovascular cause, followed by the infectious one. PD patients showed a lower rate of hospital admissions than patients in HD, and the like has been described in other series of patients transplanted.30 In the PD group there was a higher percentage of admissions due to cardiovascular causes than in the HD group, and the latter presented a higher number of admissions due to graft intolerance than the PD group. In PD, the greater dependence on RRF to maintain adequate dialysis, the greater difficulty in maintaining said RRF in the transplanted graft, and the high peritoneal transport that has been described in these people31,32 could have played a fundamental role in the difficulty in volume management and thus justify the high rate of admissions for cardiovascular events, especially heart failure, compared to HD patients.
The PD group presented a lower incidence of graft intolerance and a reduced time to its appearance, but the absence of the analysis of competitive events in this section could make data interpretation difficult. We think that the lower incidence of graft intolerance in the PD group could be related to the maintenance of low doses of immunosuppression, with the intention of preserving RRF in these patients.33 On the contrary, in HD, the greater rapidity of loss of residual diuresis and the lesser dependence on it for adequate dialysis could encourage a more rapid withdrawal of immunosuppression with the risk of intolerance to the secondary graft. Unfortunately, we do not have data on the management of immunosuppression in our work to be able to confirm this hypothesis.
In our series, as in other publications,34–36 half of the patients who started dialysis after graft failure were included again on the waiting list and 15.4% received a subsequent transplant. The mean time to re-transplantation was 26 ± 24 months (median 22 months), similar to that reported by Portolés and Marcén in their publications on transplant patients starting dialysis.22,37 In multivariate analysis, younger age of the patient proved to be the only variable that predicted access to a new transplant, as occurs in the other series.7 We do not have data regarding immunological factors such as the rate of sensitization to human leukocyte antigens (HLA) that could have a significant influence on the possibility of retransplantation.
One of the fundamental limitations of the study has been the diverse origin of the patients. The PD group was obtained from different hospitals, while all the subjects in the HD group belonged to a single hospital. This diversity in the clinical action protocols regarding fundamental aspects such as the time to start dialysis, the admission criteria, the management of the graft intolerance syndrome or the availability of PD could increase the biases of our study. Another important limitation has been the difference in survival between the two groups. The mean follow-up in the PD group was only 18 months compared to 35 in the HD group. Therefore, with the results of our study, we can affirm that PD is beneficial over HD in the first year and a half after starting dialysis.
ConclusionsWith the aforementioned limitations, this is the first study at the national level that has shown that PD compared to HD is associated with better survival in patients who start dialysis after graft failure, as well as a lower rate of hospitalization during the first year and a half after the start of the procedure. This should make us rethink the current inertia of referring these patients to HD without considering PD. We need prospective studies to confirm this result, as well as others to understand the role that maintenance of immunosuppression plays on residual renal function in transplant patients after graft failure.
Key concepts
- •
When the kidney graft fails and patients must return to dialysis, the best dialysis technique is unclear.
- •
In our series, PD was associated with lower mortality and a lower rate of hospital admissions than HD during the first year and a half on dialysis after kidney graft failure.
- •
The independent risk factors that increased mortality after the start of dialysis were, in addition to the dialysis modality, age and the use of a non-optimal access for dialysis.
- •
In the entire series, the incidence of graft intolerance syndrome was 25%, being lower in PD patients.
This work has not received any type of funding.
C. Gómez Roldán and A. Ortega Cerrato (General University Hospital of Albacete, Albacete, Spain); M.D. Albero (Virgen de los Lirios Hospital, Alcoy, Alicante, Spain); J. Pérez-Contreras and E. Muñoz de Bustillo (General University Hospital of Alicante, Alicante, Spain); J.M. Graña (Hospital de la Ribera, Alcira, Valencia, Spain); A. Seores and A. Arlandis (General Hospital of Castellón, Castellón, Spain); C. Hernaiz (Hospital de Cuenca, Cuenca, Spain); E. Bosque (Hospital General de Elda, Elda, Spain); V. Mascarós and C. Climent (Hospital Francisco de Borja, Gandía, Valencia, Spain); M. Lanuza and A. Martínez (Hospital Virgen de la Arrixaca, Murcia, Spain); M. González (University Clinical Hospital, Valencia, Spain); J.M. Escobedo, M. Montomoli and M. Giménez (General University Hospital, Valencia, Spain); A. Soldevila and R. Devesa (La Fe University Hospital, Valencia, Spain); J.C. Alonso (Hospital Luis Alcañiz, Játiva, Valencia, Spain); S. Beltrán Catalán and B. Vizcaíno Castillo (Dr. Peset University Hospital, Valencia, Spain); E. Torregrosa (Hospital de Manises, Valencia, Spain); I. Millán and S. Ros (Hospital de Elche, Alicante, Spain); V. Ramos (Denia Hospital, Alicante, Valencia); B. Diez (Torrevieja Hospital, Alicante, Spain); D. Manzano (Hospital de Cartagena, Cartagena, Murcia) and V. Andronic (Hospital de Vinalpó).
Members of Grupo Levante de Diálisis Peritoneal are listed in Appendix section.
Please cite this article as: Beltrán Catalán S, Sancho Calabuig A, Molina P, Vizcaíno Castillo B, Gavela Martínez E, Kanter Berga J, et al. Impacto de la modalidad de diálisis sobre la morbimortalidad tras el fracaso del injerto renal: análisis con eventos competitivos. Nefrologia. 2021;41:200–209.