IgA-dominant postinfectious glomerulonephritis (IgA-PIGN), a morphological variant of PIGN, is increasingly common in our setting. Unlike classical post-streptococcal PIGN, which involves C3 and IgG or only C3 deposition, IgA is the sole or dominant immunoglobulin in IgA PIGN.1 Most cases are concurrent with a Staphylococcus aureus infection, especially cutaneous, although they are also associated with coagulase-negative staphylococci and, rarely, gram-negative bacilli. IgA-PIGN has a predilection for older men, with various comorbidities, mainly diabetes mellitus, but also tumours, alcoholism or HIV infection.2 IgA-PIGN is more aggressive than the standard variant, has a poor prognosis, and in the majority of cases (70–80%) starts with acute kidney injury, proteinuria, haematuria and hypocomplementaemia. The differential diagnosis should be made with post-streptococcal PIGN and with IgA nephropathy.3 Treatment is based on the eradication of the infection and general support measures, there being little evidence to justify immunosuppressive treatment.
We present the case of a 70-year-old man with IgA PIGN secondary to cutaneous MRSA infection, which began with severe acute kidney injury. His medical history was only significant for hypertension and a traumatic right fibular fracture associated with compartment syndrome and rhabdomyolysis 5 months earlier, which had required several sessions of isolated haemodialysis, with recovery of renal function (at discharge, creatinine was 1.09mg/dl).
He was admitted to the Plastic Surgery Department 5 months later due to infection of the wound and MRSA osteomyelitis in the right foot, which was complicated by a Pseudomonas aeruginosa superinfection. Antibiotic treatment was started with linezolid and piperacillin–tazobactam. At 2 weeks, he began to present foveal oedema in the legs, hypertension, progressive volume overload, macroscopic haematuria, and foamy urine. These symptoms were associated with acute kidney injury, nephrotic-range proteinuria, and a tendency to hyperkalaemic metabolic acidosis. A renal ultrasound was performed, which showed kidneys of normal size and morphology, with adequate cortical differentiation. Serologies and the immunological study were completely negative (normal complement levels). Given the suspicion of a nephritic syndrome secondary to PIGN, the patient was initially managed conservatively with antibiotics, intravenous diuretics and antiproteinuric therapy with RAAS blockers. However, despite the good evolution of the infection, the deterioration of renal function persisted with creatinine levels of 3.54mg/dl, microscopic haematuria and nephrotic range proteinuria (with a protein/creatinine index of 5.36mg/mg), so it was decided to start treatment with corticosteroids at a dose of 1mg/kg (prednisone 60mg) and perform a renal biopsy.
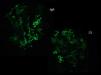
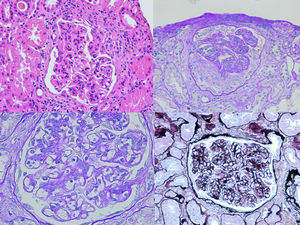
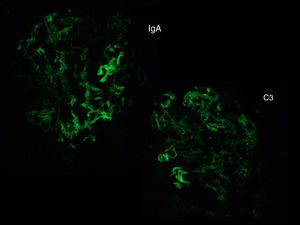
The biopsy was compatible with IgA PIGN; it showed a global and diffuse expansion of the mesangial matrix, with a focal increase in mesangial cellularity, as well as an exudative pattern of the glomerular capillary loops. In addition, 17% of the glomeruli showed an extracapillary proliferation with cellular crescents (Fig. 1). In the direct immunofluorescence test, a granular pattern was observed at the mesangial level and in the walls of the glomerular capillaries, IgA (+++) and C3 (++), without restriction of light chains, being negative for IgG, IgM, C1q and fibrinogen (Fig. 2). Although in our case it was not done, in IgA PIGN, electron microscopy usually shows electron-dense subepithelial “humps” and mesangial deposits.1,4
Evolution with corticoid treatment was adequate, with a decrease in proteinuria (protein/creatinine index of 0.7mg/mg), good blood pressure control, resolution of oedema and improvement of renal function (creatinine level at discharge was 2.08mg/dl), although microscopic haematuria persisted. After completing 8 weeks of antibiotic treatment for osteomyelitis and performing a graft in the wound area, he was discharged on a tapering corticosteroids regimen.
Based on the data available in the literature, there is no evidence for the use of steroids in the treatment of IgA PIGN. In fact, the use of immunosuppressants can increase mortality in patients with active infection. Therefore, initial management should be based on the aetiological treatment with antibiotic therapy and general measures for the control of oedema and blood pressure, following the same regimen as in similar diseases (salt restriction, loop diuretics and use of RAAS blockers).1,5
However, treatment with corticosteroids should be considered in IgA PIGN that presents with acute kidney injury and with no improvement after adequate antibiotic treatment. Only a few case series have been published in which steroid treatment improves renal prognosis.6,7 In the case presented here, after the failure of antibiotic therapy, an acceptable response to steroid treatment was observed, with progressive improvement in renal function.
Please cite this article as: Carbayo J, Rodriguez-Benitez P, Diaz-Crespo F, Muñoz de Morales A. Glomerulonefritis postinfecciosa IgA dominante secundaria a infección cutánea por Staphylococcus aureus resistente a meticilina. Nefrologia. 2019;39:446–448.