In conditions of acute ischaemic heart disease, both American and European guidelines recommend double antiplatelet therapy with ticagrelor or prasugrel and acetylsalicylic acid together with administration of high or moderate intensity statins.1 The risk of rhabdomyolysis with statins is considered to be 1/105 patients/year,2 although the risk of myopathy is 1/103–104/patients/year, and it is multiplied 5 times if 2 drugs are combined.3 We present a case of rhabdomyolysis in relation to atorvastatin and ticagrelor.
A 69-year-old woman with preserved renal function. Chronic type 2 diabetes mellitus, without diabetic retinopathy or nephropathy. Severe chronic ischaemia of the lower limbs. Hypertension, morbid obesity and mix dyslipidaemia. Chronic consumer of NSAIDs. In treatment with insulin, ARBs, thiazide and ibuprofen. She was admitted for Killip III NSTEMI due to non-revascularisable 3-vessel disease. After optimising medical treatment, she improved slowly despite several infectious complications and severe deconditioning syndrome. At 4 weeks, without any triggering trauma, she developed generalised muscle pain with CPK levels of 27,000U/L. During the previous week, she had been treated with 90mg/day of ticagrelor, omeprazole, paracetamol, 40mg/day of atorvastatin, amlodipine and duloxetine. Viral serology, thyroid profile, ACTH, cortisol, complete immunology, tumour markers, paracetamol levels, vitamin B12 and serum folic acid were all normal. There were no symptoms of serotonin syndrome or acute adrenal insufficiency. All drugs were discontinued and clopidogrel was added to the treatment. The patient developed heart failure with oliguric renal failure and hyperkalaemia, which required 4 sessions of acute haemodialysis. She quickly regained renal function and improved clinically, but on the eighth day she had a new coronary syndrome resulting in her death within 48h.
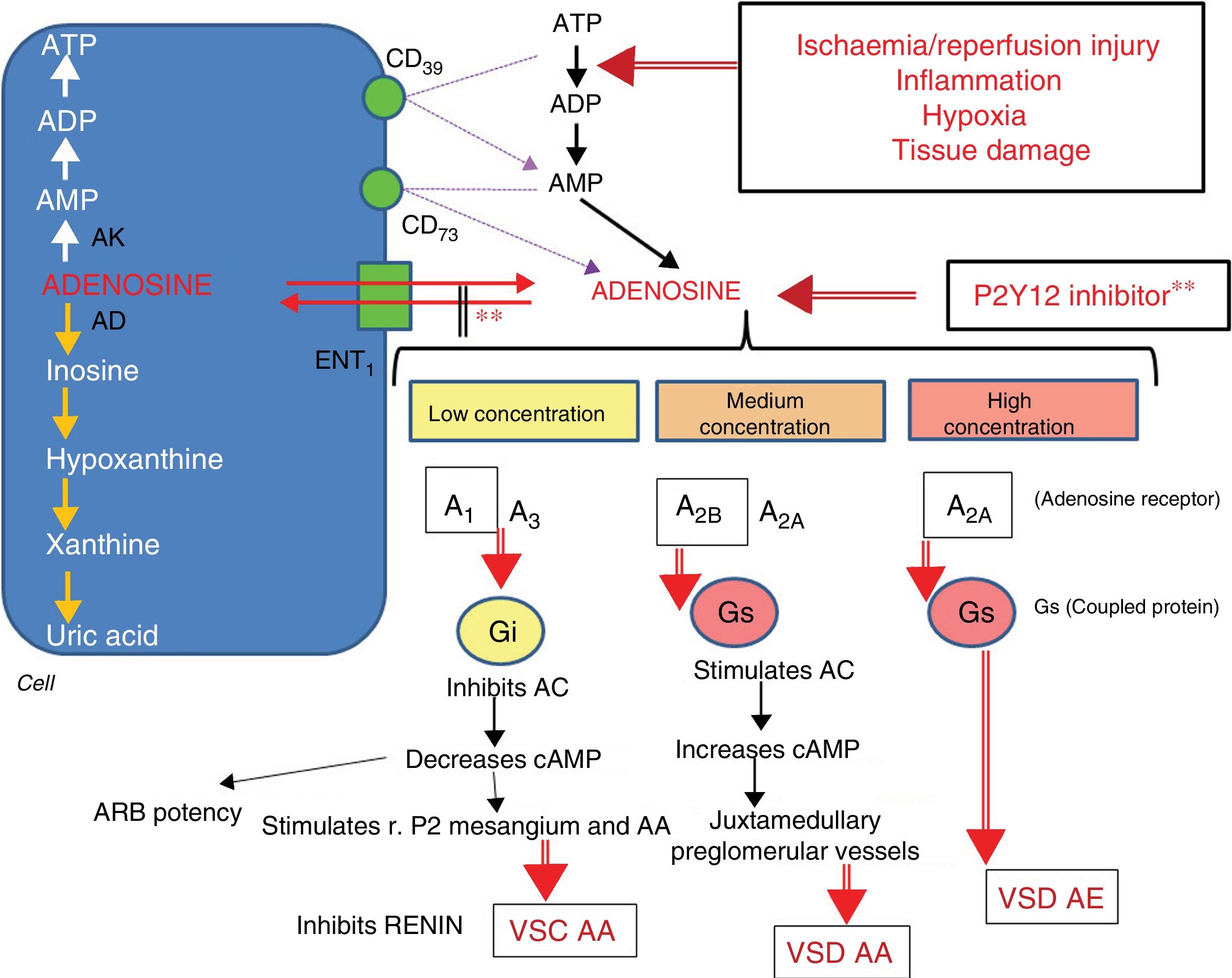
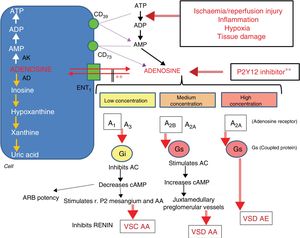
We present the case of a multi-pathological patient who suffered severe rhabdomyolysis with renal failure that required kidney replacement therapy, probably caused by the interaction between ticagrelor and atorvastatin (8 points on the Horn interactions scale4 and 7 in the Naranjo adverse drug reaction probability scale5). This is not an unusual situation. Interaction between 2 drugs that cause serious adverse effects is responsible for 2.8% of hospital admissions.1 Ticagrelor is absorbed linearly, reaching peak plasma concentration after 1–4h, and has an elimination half-life of 7h for the active substance and 9 for its active metabolite (AR-C124910XX). Over half (58%) is eliminated6 in the faeces, and 27% is eliminated in the urine as an inactive metabolite. Its mechanism of action is the reversible inhibition of platelet aggregation by inhibiting the P2Y12 receptor activity; this takes place by means of inhibition of the ENT1 cellular transporter, which reduces cellular adenosine uptake and induces an increase in ticagrelor plasma levels.7–12 As shown in the Platelet Inhibition and Patient Outcome (PLATO) study,13 it decreases cardiovascular mortality in acute ischaemic heart disease compared to clopidogrel, presumably due to prolongation of the action of atorvastatin, since both are metabolised by the CYP 450 3A4/3A5 cytochrome. The atorvastatin/ticagrelor interaction is considered a minor risk,1 since its co-administration only increases 1.4 times the AUC concentration (area under curve of the maximum plasma concentration of drug in relation to time). Therefore, it is considered a reasonable combination, and dose limitation is not recommended. Why could rhabdomyolysis have occurred in this case? There could be multiple causes. First of all, the possible polymorphism of CYP 450 3A4/3A5, which could lead to an increase in blood concentration of the statin, and the combination with amlodipine, which is a weak inhibitor of CYP3A4 and CYP3A5 and increases the AUC of atorvastatin by 18%8 and induces the accumulation of active ticagrelor metabolites. Second, due to the renal action of ticagrelor. The PLATO study showed that ticagrelor induced a 30% increase in creatinine in more than 25% of patients, mainly those who with previous renal insufficiency, aged over than 75 years, and in previous treatment with ACEI. Adenosine is synthesised after ADP is metabolised by nucleotidases CD39 and CD73. Its levels increase in plasma following cellular stress, ischaemia/reperfusion or inflammation. It is rapidly captured by the cells through the ENT and CNT transporters (equilibrium and concentrative nucleoside transporters). It has a general vasodilating action, but in the kidneys its action depends on its concentration, and it can be vasoconstrictive, which is essential in the glomerular tubular feedback mechanism, or vasodilator. At low concentrations, it stimulates receptor 1 (and to a lesser extent 3), which causes the inhibition of adenylyl cyclase, and cAMP, potentiating the effect of angiotensin II and inducing vasoconstriction (VSC) of the afferent arteriole (AA) by stimulating purinergic receptors (P2) of mesangial cells and AA and inhibition of renin secretion, which helps maintain the autoregulation of renal blood flow (RBF).11,12 At higher doses, receptor 2, mainly 2B, is stimulated. This is expressed in juxtamedullary preglomerular vessels, and increases the concentration of cAMP, inducing vasodilatation (VSD) of the AA and reducing the efficacy of the autoregulatory mechanism. Even at higher concentration, the predominant effect is stimulation of 2A receptors with VSD of the efferent arteriole (EA), which causes a reduction in FSR and the glomerular filtration rate (GFR) (Fig. 1). In these cases, it is recommended to replace atorvastatin with fluvastatin, which is metabolised by P459 CYP 2C9, and ticagrelor with clopidogrel. Several similar cases have been described in the literature6,8,9,14–16 with ticagrelor and different statins used at adequate doses. In the majority, the clinical picture presents after a period of 1–3 months of use of these drugs. In our case, both the increased adenosine due to inhibition of the ENT1 transporter and the stimulus due to ischaemic injury could have caused renal damage with accumulation of the statin, even though the doses used were correct. This compels us to reassess this recommendation in situations of polypharmacy, clinical instability or special fragility of patients.
Metabolism of adenosine and actions depending on its concentration. Adenosine enters and leaves the cell by means of the ENT1 transporter ATP is transformed into AMP by nucleotidase CD39, and this is transformed into adenosine by nucleotidase CD73. Ischaemia/reperfusion, inflammation, hypoxia and tissue injury accentuate the transformation of ATP to ADP. P2Y12 inhibitors increase adenosine levels. Adenosine at low concentration stimulates its A1 and, to a lesser extent, A3 receptors, which leads to vasoconstriction (VSC) of the afferent arteriole (AA). At half concentration, A2B receptors are stimulated and, to a lesser extent, A2A, which leads to vasodilatation (VSD) of AA. At high concentration, the A2A receptor is mainly stimulated and the predominant effect is VSD efferent arteriole (EA). AC: adenylyl cyclase; AD: adenosine deaminase; ADP: adenosine diphosphate; AK: adenosine kinase; AMP: adenosine monophosphate; ARB: angiotensin receptor blocker; ATP: adenosine triphosphate; ENT1: equilibrative nucleoside transporter 1; GLM: glomerulus; r.P2: type 2 purinergic receptor. ** Inhibitor P2Y12 inhibits the ENT1 transporter.
This article was not funded by any organisation.
Conflicts of interestThe authors declare they have no conflicts of interest.
Please cite this article as: Martín Navarro JA, Gutiérrez Sánchez MJ, Petkov Stoyanov V, Jiménez Herrero MC. Fracaso renal agudo secundario a rabdomiólisis en paciente en tratamiento con ticagrelor y atorvastatina. Nefrología. 2019;39:448–450.