La hipercalcemia es un efecto adverso potencial de las resinas cálcicas de intercambio iónico, de uso frecuente en el tratamiento y la prevención de la hiperpotasemia en la enfermedad renal crónica (ERC). Describimos una serie de siete pacientes con ERC moderada de la consulta de Nefrología Clínica (filtrado glomerular medio estimado por CKD-EPI: 41,29 ± 10,83 ml/min/1,73 m2), que presentan hipercalcemia leve en relación con el tratamiento con poliestireno sulfonato cálcico. El calcio sérico se elevó de media 0,91 ± 0,46 mg/dl, con un descenso paralelo de los niveles de hormona paratiroidea intacta (iPTH) de 49,29 ± 52,24 ng/dl de media. Tras la retirada o la reducción de la dosis, se objetivó una recuperación de las cifras de calcio e iPTH séricos. Los quelantes cálcicos de potasio se deben incluir en el diagnóstico diferencial de la hipercalcemia en pacientes con ERC no avanzada.
Hypercalcaemia is a potential adverse effect of calcium-containing ion exchange resins, often used in the treatment and prevention of hyperkalaemia in chronic kidney disease (CKD). We describe a series of seven outpatients with moderate CKD (mean glomerular filtration rate estimated with the CKD-EPI formula: 41.29±10.83mL/min/1.73m2), presenting mild hypercalcaemia in relation to the treatment with calcium polystyrene sulfonate. Serum calcium increased a mean of 0.91±0.46mg/dL, with a mean concomitant decrease of serum intact parathormone (iPTH) of 52.24±49.29ng/dL. After treatment withdrawal or dose reduction, we observed a recovery of serum calcium and iPTH values. Treatment with calcium-based potassium binders should be included in the differential diagnosis of hypercalcaemia in patients with moderate CKD.
INTRODUCTION
Hyperkalaemia is one of the most frequent electrolyte abnormalities in chronic kidney disease (CKD) patients. It affects approximately 2.5% of CKD patients older than 651 and it becomes symptomatical with a prevalence of 1.5% in the hospitalised population.2 The main cause of hyperkalaemia in CKD is the decrease of glomerular filtration rate, but there often are other contributing factors, such as the use of hyperkalaemia-inducing drugs (renin-angiotensin-aldosterone system blockers, nonsteroidal anti-inflammatory drugs, etc.). In addition, some causes of CKD, such as diabetic nephropathy or tubulointerstitial nephritis, can convey hyporeninemic hypoaldosteronism that favours the occurrence of hyperkalaemia with moderately decreased glomerular filtration rates.3
Severe hyperkalaemia is a proarrhythmogenic condition, which can produce bradycardia, ventricular fibrillation and ultimately death.4.5 Therefore, we must use various methods in order to control it. A low potassium diet is one of the first steps to implement by explaining to the patient the need to moderate the intake of fruits and raw vegetables, chocolate or nuts, and also by recommending the double boiling of vegetables and legumes.
If the trend to hyperkalaemia persists, it is suitable to prescribe ion exchange oral resins, which act as potassium “binding agents”.6 The most widely used in our setting is calcium polystyrene sulfonate (CPS), although there are others such as sodium polystyrene sulfonate.7
The most common adverse effects of CPS are hypokalaemia, in patients compliant with the restrictive diet, and severe constipation, which can sometimes result in intestinal occlusions.8.9 CPS owes its binding power to its calcium content, since it exchanges a calcium ion for two potassium ions in the gastrointestinal tract. A certain amount of this calcium can be absorbed, which is not a problem for patients with more advanced stages of kidney disease, who tend to present hypocalcaemia. Nonetheless, in earlier stages the level of serum calcium may mildly increase, consequently decreasing the levels of serum intact PTH (iPTH). Resincalcio® and Sorbisterit® are the two CPS preparations available in Spain. Their technical data sheets indicate hypercalcaemia as a possible adverse effect.10 The Spanish Society of Nephrology’s guidelines for the management of bone mineral metabolism mentions the importance of remembering the calcium content of the ion exchange resins.11 It is estimated that one gram of CSP contains 2.1 mmol of pure calcium element,12 so that a 15 grams pack of Resincalcio® adds 31.7 mmol of pure calcium element. A Mexican study makes a brief reference to increases of calcium absorption due to use of these drugs, though it is not statistically significant.13 There are no other data described in the literature concerning this possible adverse effect.
MATERIAL AND METHOD
In 2011, we identified a patient with mild hypercalcaemia in the Nephrology Department of the Gregorio Marañón General University Hospital, receiving outpatient services at the time. Other known causes of hypercalcaemia were ruled out, including contributions of exogenous calcium or vitamin D, and concomitant bone disease. There was a possibility of hypercalcaemia being related to the treatment with resins (Resincalcio®) and as a result we proceeded to withdrawal. Hypercalcaemia correction was observed and it was reported as an adverse effect.
Subsequently, for the next 3 months we collected all patients on follow-up by our Clinical Nephrology Unit (which excludes advanced CKD with glomerular filtratation rate <20mL/min/1.73m2), who showed elevations of serum calcium with no attributable secondary causes other than the contributions of potassium binders. In these patients, we discontinued CPS treatment or reduced doses. We collected data relative to renal function, calcaemia and serum iPTH levels, prior to the introduction of the binder, during treatment and after withdrawal.
For the literature review, we carried out a search in the Pubmed-Medline database with the MeSH (Medical Subject Heading) terms «polystyrene sulfonic acid» and «calcium polystyrene sulfonate»; both independently and in combination with other MeSH terms: “Hyperkalaemia”, “hypercalcaemia” and “chronic kidney disease”.
RESULTS
Seven patients were found to meet the criteria described above. Six men and a woman, between the ages of 50 and 89 years (mean: 66 years). Three patients (43%) are diabetics; two (28.5%) have chronic interstitial nephropathy; one (14.3%) has systemic lupus erythematous, and the other one, chronic glomerulonephritis. The average glomerular filtration rate estimated with the CKD-EPI formula is 41.29±10.83mL/min/1.73m2. Four patients were classified in stage 3A, two in stage 3B and one in stage 4 of the K/DOQI classification guidelines. 85% (6 patients) were treated with renin-angiotensin system blockers for the control of arterial hypertension or proteinuria. The serum potassium level prior to treatment was 5.9±0.46mmol/L. The average dose of Resincalcio® was 8.93±3.49g/day.
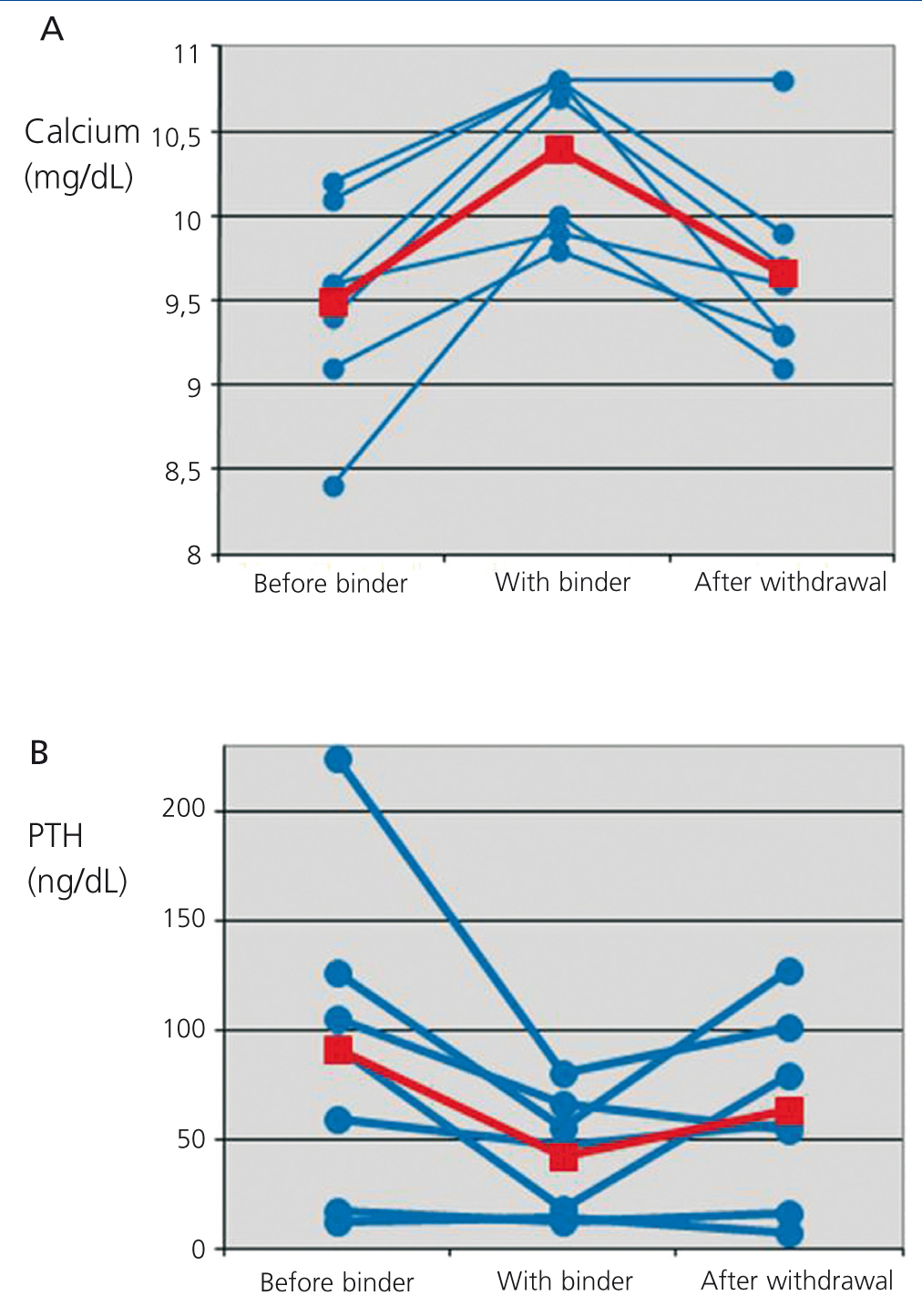
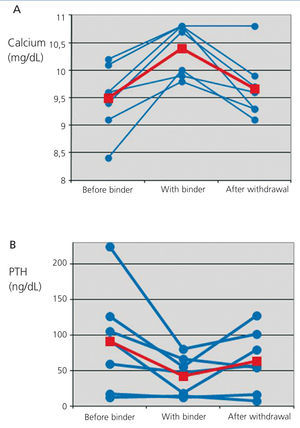
Serum calcium rose on average 0.91±0.46mg/dL over the baseline levels. Besides, there was a parallel decrease in the levels of iPTH of 49.29±52.24ng/dL, equivalent to an average decrease of 38.83% over baseline levels.
Treatment with CPS was interrupted or dose was reduced at the nephrologist's criterium. In the subsequent follow-up, calcium and iPTH levels returned to baseline levels in most patients (Figure 1, Table 1).
Given that this adverse effect is not clearly described in the literature, those cases with overt biochemical hypercalcaemia were reported to the Drug-monitoring Centre of Madrid, through the Spontaneous Reporting of Adverse Drug Reactions Programme (“Yellow Card”).
DISCUSSION
Hypercalcaemia associated to treatment with ion-exchange calcium resins is not frequent in the clinical setting, probably because potassium binders are only used in advanced stages of CKD. In these stages we find hyperkalaemia with tendency to hypocalcaemia and secondary hyperparathyroidism, each effect counteracting the other with a final benefit for the patient.
Occasionally, we find patients with a tendency to hyperkalaemia despite being on early stages of renal dysfunction. This is due to the existence of certain pathologies that produce hyperkalaemia through hyporeninemic hypoaldosteronism, such as diabetes or chronic interstitial nephritis. We usually need to prescribe various hyperkalaemia-inducing drugs, such as beta blockers or reninangiotensin-aldosterone system blockers, often at full doses, so it is possible to see patients with mild or moderate renal dysfunction and hyperkalaemia that require therapeutic actions.
In these situations, patients have normal levels of serum calcium. Therefore, we must take into account the potentially hypercalcaemic effect of CPS, and include it in the differential diagnosis. In our study, all patients presented glomerular filtration rate greater than 25mL/min/1.73m2, and four of them reached biochemical hypercalcaemia. In one case, this hypercalcaemia persisted because it was considered necessary to maintain the treatment with CPS.
With the rise of blood calcium levels we observed a decrease of iPTH. Under normal conditions, with normal or slightly elevated iPTH levels, this effect is trivial or even beneficial. Nonetheless, as shown in Figure 1, two patients had iPTH values <20ng/dL. In this situation, a stronger inhibition of the parathyroid gland may turn into a clinical problem. These variations of iPTH also are reversible with the withdrawal of CPS.
CONCLUSION
In patients with moderate chronic kidney disease, treatment of hyperkalaemia with ion-exchange calcium resins (CPS) can produce mild hypercalcaemia, which must be taken into account in the differential diagnosis.
Conflicts of interest
The authors declare that they have no potential conflicts of interest related to the contents of this article.
Table 1. Mean of serum calcium and parathormone before, during and after treatment with Resincalcio®
Figure 1. Evolution of serum calcium (A) and parathormone (B) levels before the prescription of calcium polystyrene sulfonate, during treatment and after withdrawal