Despite the frequency with which anemia is present in patients with chronic kidney disease (CKD), its relationship with gastrointestinal lesions has not been studied.
MethodA cross-sectional, analytical, observational study involving one year of recruitment was carried out to determine the prevalence of endoscopic gastrointestinal lesions and associated risk factors in asymptomatic patients with chronic kidney disease stages 1–5 and anemia who had a positive qualitative immunochemical fecal occult blood test.
ResultsA total of 9658 patients with CKD were analyzed, of which 286 (2.9%) had anemia; 198 had a positive fecal occult blood test (47% male, 71.1±11.8 years). The endoscopic study revealed 255 lesions, with at least one lesion in 68.2% of patients, with the most prevalent being: adenomatous colorectal polyps (39.6%), acute lesions of the gastric mucosa (22.6%), neoplastic lesions 15.1%), angiodysplasia (14.4%), oesophagitis (8.4%), inflammatory bowel disease (4.8%) and ischemic colitis (3.1%). Uremia and acetylsalicylic acid were identified as risk factors for acute gastric mucosal lesions. Angiodysplasia was associated with alcoholism, a more advanced stage of chronic kidney disease, anemia, and lack of response to erythropoiesis-stimulating agents. Age and refractory anemia were risk factors for adenomatous polyps and colorectal cancer.
ConclusionRenal patients with anemia could benefit from an endoscopic study due to their high prevalence of gastrointestinal lesions, particularly adenomatous polyps and colorectal cancer, which are more common in those over 50 years of age with CKD stages 3–5.
A pesar de la frecuencia con que la anemia está presente en los pacientes con enfermedad renal crónica (ERC), su relación con lesiones gastrointestinales no ha sido estudiada.
MétodoEstudio observacional analítico transversal de un año de reclutamiento para determinar la prevalencia de lesiones gastrointestinales endoscópicas y los factores de riesgo asociados en pacientes asintomáticos con ERC estadios 1-5 y anemia que presentaban un test inmunoquímico cualitativo de sangre oculta en heces positivo.
ResultadosSe analizaron 9.658 pacientes con ERC, de los que 286 (2,9%) presentaban anemia; 198 tuvieron un test de sangre oculta en heces positivo (47% varones, 71,1±11,8 años). El estudio endoscópico reveló 255 lesiones, con al menos una lesión en el 68,2%, siendo las más prevalentes: pólipos colorrectales adenomatosos (39,6%), lesiones agudas de la mucosa gástrica (22,6%), lesiones neoplásicas (15,1%), angiodisplasias (14,4%), esofagitis (8,4%), enfermedad inflamatoria intestinal (4,8%) y colitis isquémica (3,1%). La uremia y el ácido acetilsalicílico fueron identificados como factores de riesgo de lesiones agudas de la mucosa gástrica. Las angiodisplasias se relacionaron con el enolismo, el mayor estadio de ERC, la anemia y la ausencia de respuesta a agentes estimulantes de la eritropoyesis. La edad y la anemia refractaria constituyeron factores de riesgo de pólipos adenomatosos y cáncer colorrectal.
ConclusiónLos pacientes renales con anemia podrían beneficiarse de un estudio endoscópico debido a la alta prevalencia de lesiones gastrointestinales que presentan, particularmente pólipos adenomatosos y cáncer colorrectal, más frecuentes en los mayores de 50 años con ERC estadios 3-5.
Anemia is a common complication of chronic kidney disease (CKD), and the severity of anemia increases with the worsening of the renal function.1 As the disease progresses, the production of erythropoietin decreases, with the consequent reduction in the formation of red blood cells in the bone marrow. However, the generation of anemia in CKD may be caused by more than one factor such as deficiency of iron, folic acid or vitamin B12, hemolysis, gastrointestinal losses and myelodysplastic syndromes. Also, there are factors that decrease the response to erythropoietin.
Additionally, uremia produces alterations at different levels of the gastrointestinal system and, although its pathogenesis is not known, there is an increased prevalence of gastrointestinal lesions in individuals with CKD.2 It is estimated that a 40–90% of patients with CKD have endoscopic abnormalities3; the most prevalent are: acute lesions of the gastric mucosa (ALGM),4–6 angiodysplasias,7 diverticulosis,8 colonic polyposis and colorectal cancer.9,10 The most frequently described gastrointestinal disease is angiodysplasia, but the most serious is colorectal cancer which modifies the prognosis in terms of quality of life and survival. It has been suggested that the association between CKD and cancer has its origin in immune alterations caused by uremia.11,12
According to the International Agency for Research on Cancer of the World Health Organization,13,14 colorectal cancer is the third most common in the world (after the lung and breast), with about 1.4 million new cases diagnosed in 2012. Around 54% of cases of colorectal cancer occur in more developed countries. The highest incidence of colorectal cancer is found in Oceania and Europe, and the lowest incidence in Africa and Asia. Mortality during the first year is 8.5%, with the highest estimated rates in Central and Eastern Europe (14.9 per 100,000 for both genders), and the lowest in West Africa (3.3 per 100,000).
The usual age of presentation of sporadic colorectal cancer is between the sixth and eighth decades of life whereas in the hereditary forms the diagnosis is usually before 50 years of age.15 The clinical presentation varies depending on the location of the tumor. Within the differential diagnosis of colorectal cancer should include other gastrointestinal neoplasms and other entities such as inflammatory bowel disease, angiodysplasias, diverticulitis, actinic, ischemic or infectious colitis and intestinal tuberculosis. If colorectal cancer is suspected, it is imperative to perform a colonoscopy to confirm the presence of neoplasia and to obtain biopsies for histological diagnosis.15 Endoscopy also allows the identification of potential concomitant lesions. Regarding the screening of colorectal cancer, it has been shown that in individuals older than 50 years without a family history of colorectal cancer, performing an annual or biennial fecal occult blood test (FOBT) sigmoidoscopy every 5 years or colonoscopy every 10 years decreases the incidence and mortality due to colorectal cancer.16
The immunochemical FOBT are based on the detection of human hemoglobin by specific antibodies and have a sensitivity of 66–90% and a specificity higher than 90%.17,18 The FOBT is a non expensive, simple, non-invasive technique that is well accepted by the patient, which is not affected by the intake foods with peroxidase activity or drugs.19
The KDIGO guidelines recommend to perform an endoscopic study in patients who present a hyporesponsiveness to treatment with erythropoiesis-stimulating agents (ESA).20 Since in patients with CKD and anemia it is a priority to carry out a study to identify the origin of the anemia, rule out contributing factors and optimize the response to treatment,21–23 the objective of this study was to determine the prevalence of endoscopic gastrointestinal lesions in patients with CKD and anemia with a positive FOBT, as well as analyzing the associated risk factors.
MethodThis is an observational, analytical, cross-sectional study of one year of recruitment was carried out in the La Mancha-Centro Hospital Complex, Alcázar de San Juan, Ciudad Real, Spain.
All patients older than 18 years with CKD and anemia from the outpatient nephrology service were included. Patients excluded were: those with gastrointestinal symptoms, taking non-steroidal antiinflammatory agents, those undergoing endoscopic study in the last 5 years and those who had one or more factors of hyporesponsiveness to ESA: active infection, parathormone serum greater than 500pg/ml, aluminum poisoning, neoplasia, on chemotherapy or radiotherapy, concomitant treatment with immunosuppressive drugs or data of hemolysis.
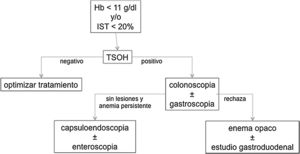
The estimated glomerular filtration rate (eGFR) was calculated by the formula of Modification on Diet on Renal Disease of 6 variables (MDRD-6) and the stages of CKD were defined according to the National Kidney Foundation.24 Anemia was defined, according to the guidelines KDIGO25 and NICE,26 as the presence of a serum hemoglobin lower than 11g/dl and/or a transferrin saturation index lower than 20%.
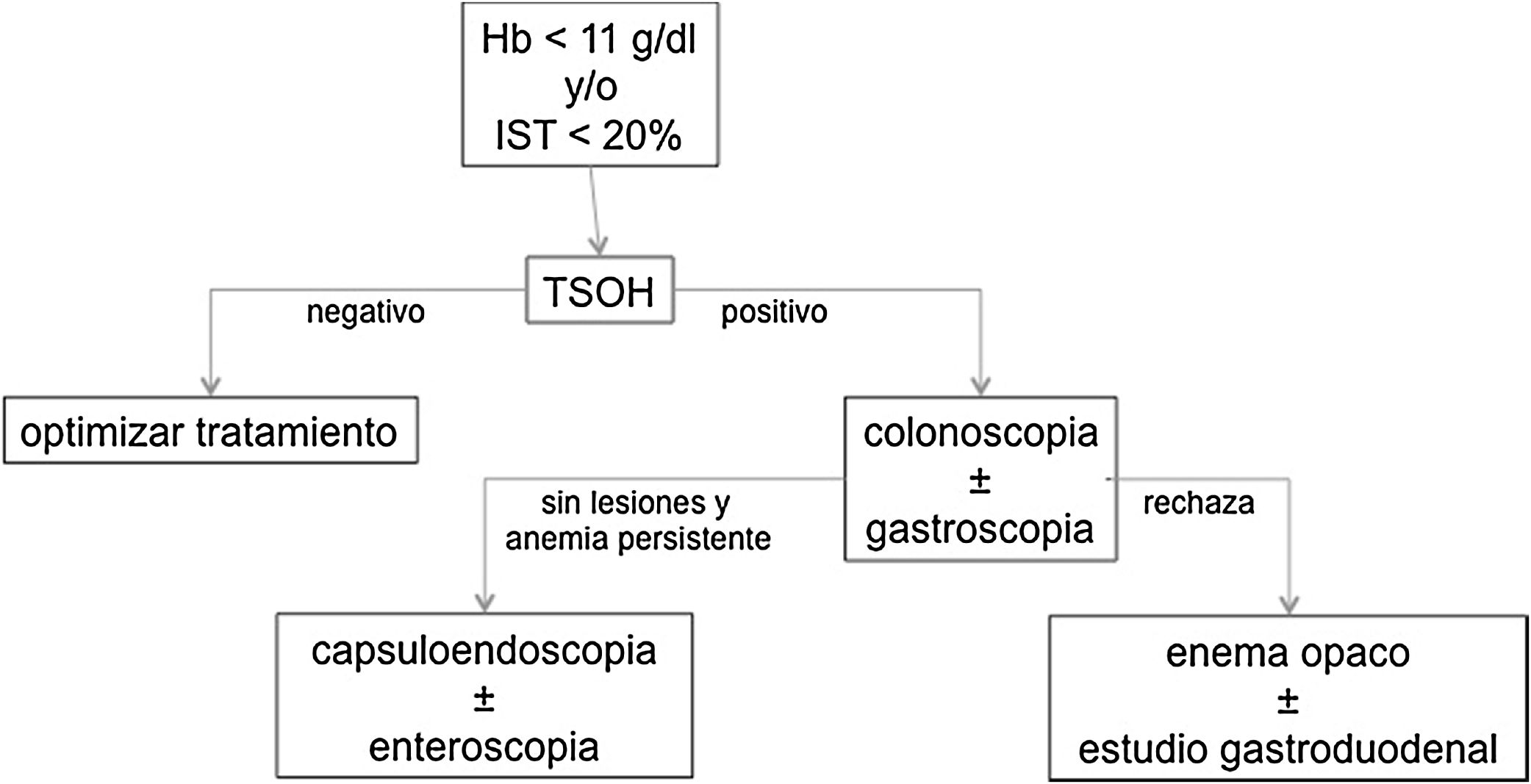
The patients had a qualitative immunochemical FOBT (DIMA®, 94.6% sensitivity, 99.3% specificity). Those with a positive result were referred to the gastrointestinal service to undergo a colonoscopy and, if there were no lesions patients underwent gastroscopy in the same act; in case of persistent anemia with initial negative endoscopic study the study was extended with capsuloendoscopy and/or enteroscopy. Patients who rejected the endoscopic study were asked for an opaque enema and, if no lesions were observed, a barium gastroduodenal study (Fig. 1). Sociodemographic and anthropometric data were collected as well as analytical parameters (blood count, biochemistry, proteinogram, vitamin B12 and folic acid), concomitant pharmacological treatment and endoscopic findings.
The procedures were carried out following the Standards of Good Clinical Practices and all the patients provided informed consent to participate in the study.
Statistic analysisA descriptive analysis of the variables included in the study was performed. The quantitative variables were summarized by measuring of central tendency (mean or median, according to the distribution of the data) and dispersion (standard deviation or interquartile range, respectively). The qualitative variables were expressed with absolute and relative frequencies (percentage).
The comparison between groups with positive and negative FOBT was determined by Chi-square test (or Fisher's exact test when necessary) for qualitative variables or Student's t-test for quantitative variables. The contrast between the different lesions and the possible associated risk factors was performed using the Chi-square test (or Fisher's exact test when necessary). The odds ratio (OR) was also calculated.
The statistical analysis was performed with the SPSS® program, version 18. In all the tests, the probability of a p<0.05 was considered statistically significant
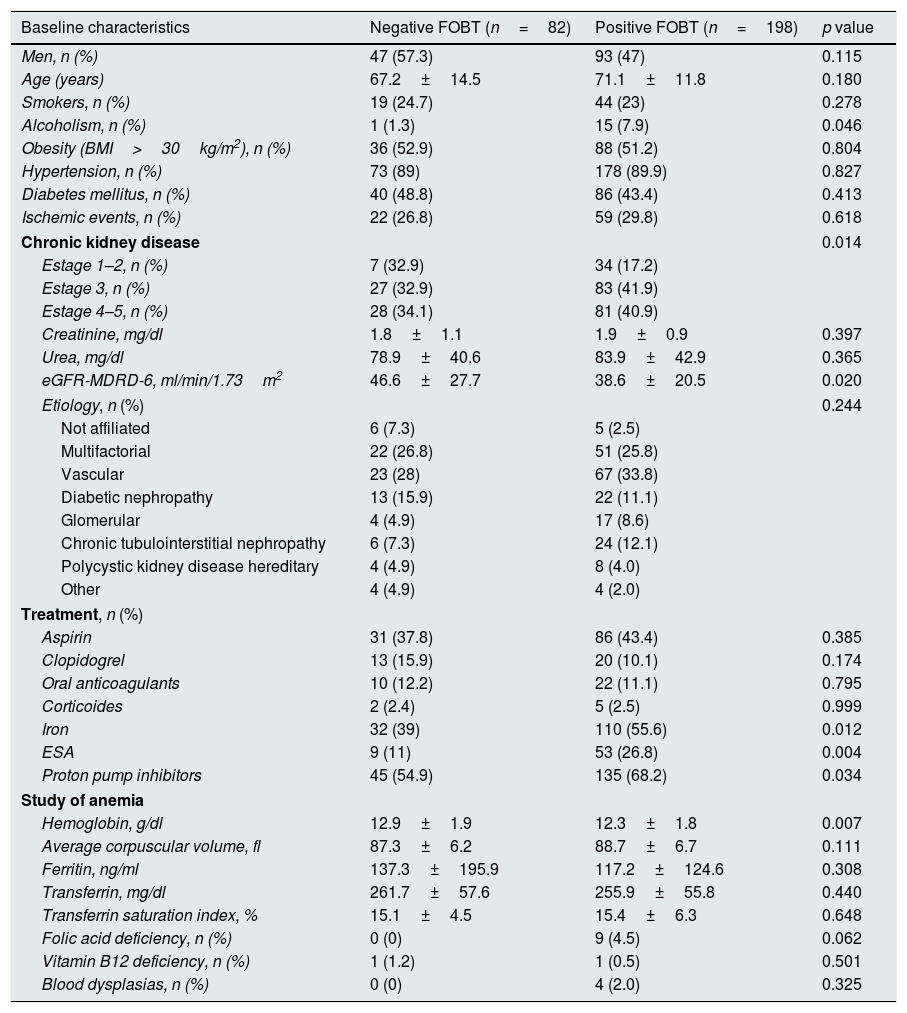
ResultsOf the 9658 patients with CKD attended during the year of recruitment, 286 (2.9%) had anemia. Of these, 6 rejected to have FOBT performed and 198 (69.2%) had a positive result, of which 47% were male, with an average age of 71.1±11.8 years, and they were derived to the gastrointestinal disease service to undergo endoscopic. The baseline characteristics of patients with anemia and with the FOBT performed, both negative and positive, are summarized in Table 1.
Baseline characteristics of patients with anemia and a FOBT.
| Baseline characteristics | Negative FOBT (n=82) | Positive FOBT (n=198) | p value |
|---|---|---|---|
| Men, n (%) | 47 (57.3) | 93 (47) | 0.115 |
| Age (years) | 67.2±14.5 | 71.1±11.8 | 0.180 |
| Smokers, n (%) | 19 (24.7) | 44 (23) | 0.278 |
| Alcoholism, n (%) | 1 (1.3) | 15 (7.9) | 0.046 |
| Obesity (BMI>30kg/m2), n (%) | 36 (52.9) | 88 (51.2) | 0.804 |
| Hypertension, n (%) | 73 (89) | 178 (89.9) | 0.827 |
| Diabetes mellitus, n (%) | 40 (48.8) | 86 (43.4) | 0.413 |
| Ischemic events, n (%) | 22 (26.8) | 59 (29.8) | 0.618 |
| Chronic kidney disease | 0.014 | ||
| Estage 1–2, n (%) | 7 (32.9) | 34 (17.2) | |
| Estage 3, n (%) | 27 (32.9) | 83 (41.9) | |
| Estage 4–5, n (%) | 28 (34.1) | 81 (40.9) | |
| Creatinine, mg/dl | 1.8±1.1 | 1.9±0.9 | 0.397 |
| Urea, mg/dl | 78.9±40.6 | 83.9±42.9 | 0.365 |
| eGFR-MDRD-6, ml/min/1.73m2 | 46.6±27.7 | 38.6±20.5 | 0.020 |
| Etiology, n (%) | 0.244 | ||
| Not affiliated | 6 (7.3) | 5 (2.5) | |
| Multifactorial | 22 (26.8) | 51 (25.8) | |
| Vascular | 23 (28) | 67 (33.8) | |
| Diabetic nephropathy | 13 (15.9) | 22 (11.1) | |
| Glomerular | 4 (4.9) | 17 (8.6) | |
| Chronic tubulointerstitial nephropathy | 6 (7.3) | 24 (12.1) | |
| Polycystic kidney disease hereditary | 4 (4.9) | 8 (4.0) | |
| Other | 4 (4.9) | 4 (2.0) | |
| Treatment, n (%) | |||
| Aspirin | 31 (37.8) | 86 (43.4) | 0.385 |
| Clopidogrel | 13 (15.9) | 20 (10.1) | 0.174 |
| Oral anticoagulants | 10 (12.2) | 22 (11.1) | 0.795 |
| Corticoides | 2 (2.4) | 5 (2.5) | 0.999 |
| Iron | 32 (39) | 110 (55.6) | 0.012 |
| ESA | 9 (11) | 53 (26.8) | 0.004 |
| Proton pump inhibitors | 45 (54.9) | 135 (68.2) | 0.034 |
| Study of anemia | |||
| Hemoglobin, g/dl | 12.9±1.9 | 12.3±1.8 | 0.007 |
| Average corpuscular volume, fl | 87.3±6.2 | 88.7±6.7 | 0.111 |
| Ferritin, ng/ml | 137.3±195.9 | 117.2±124.6 | 0.308 |
| Transferrin, mg/dl | 261.7±57.6 | 255.9±55.8 | 0.440 |
| Transferrin saturation index, % | 15.1±4.5 | 15.4±6.3 | 0.648 |
| Folic acid deficiency, n (%) | 0 (0) | 9 (4.5) | 0.062 |
| Vitamin B12 deficiency, n (%) | 1 (1.2) | 1 (0.5) | 0.501 |
| Blood dysplasias, n (%) | 0 (0) | 4 (2.0) | 0.325 |
ESA: agents that stimulate erythropoiesis; BMI: body mass index; MDRD: modification of diet in renal disease; eGFR: estimated glomerular filtration rate; FOBT: fecal occult blood test.
The presence of a positive FOBT was significantly associated to enolic habit, eGFR, serum hemoglobin and treatment with iron supplements, ESA and proton pump inhibitors.
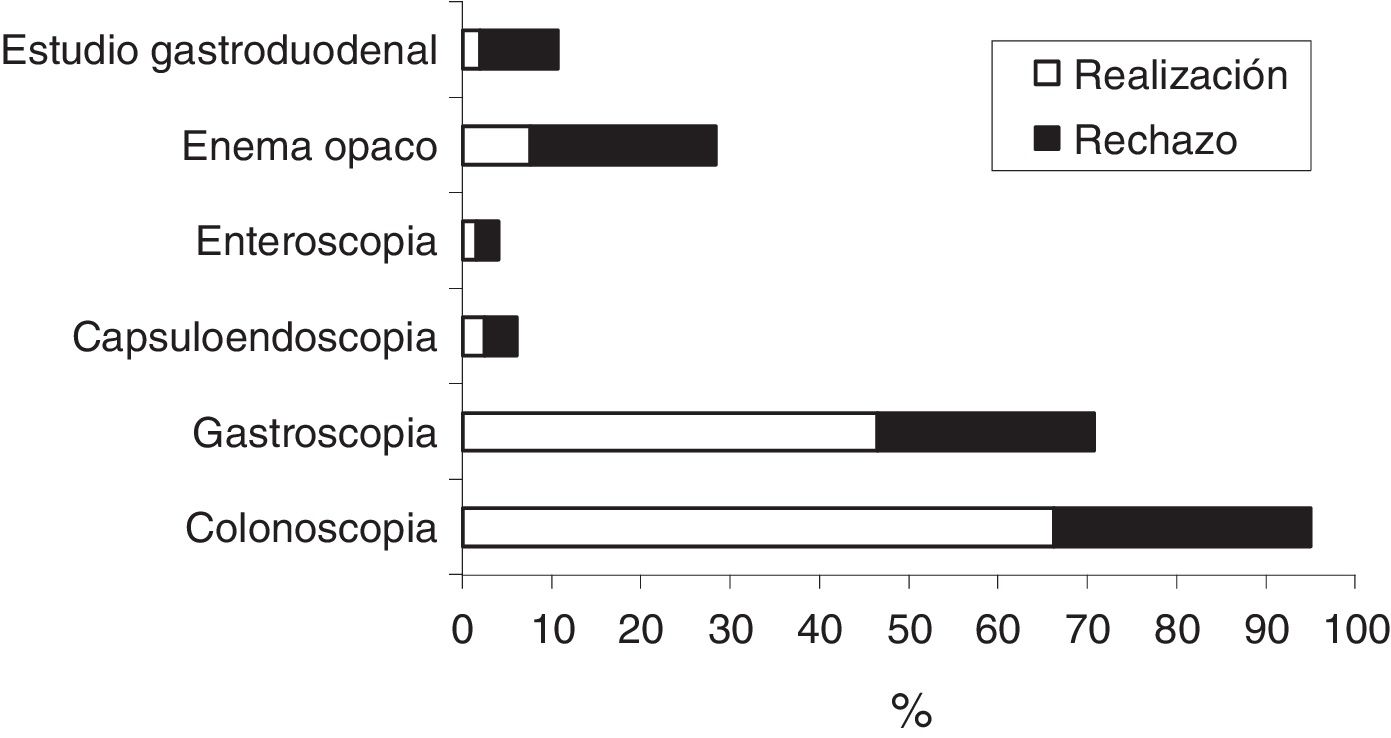
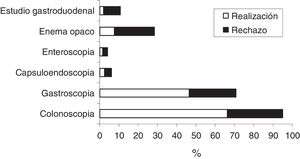
A 20.6% of patients rejected to have colonoscopy and a 17.5% refused to undergo gastroscopy. Fig. 2 shows the frequency of indication (expressed as the total sum of each bar of the histogram), acceptance and rejection of the tests to study gastrointestinal bleeding in patients with a positive FOBT.
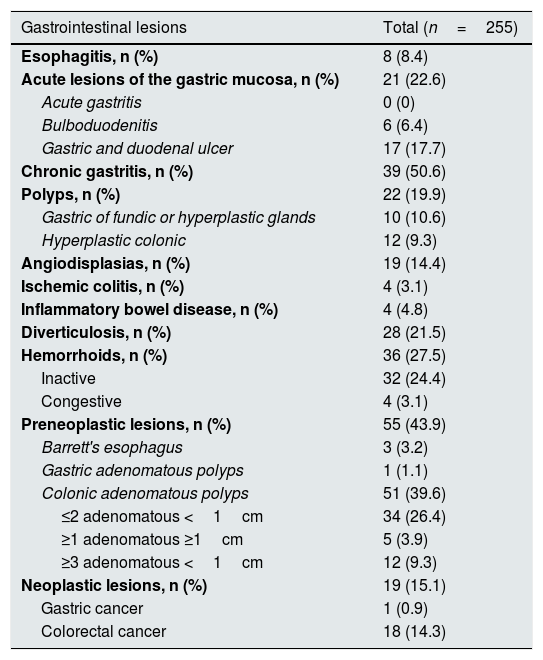
Prevalence of gastrointestinal lesionsOf the 198 patients with a positive FOBT, 68.2% presented some type injury detected in the complementary tests performed, there were a total of 255 lesions. The prevalence of gastrointestinal lesions in patients with positive FOBT is detailed in Table 2. The most prevalent endoscopic lesions were chronic gastritis (50%), preneoplastic lesions (43.9%), acute lesion of gastric mucose (ALGM)(22, 6%), diverticulosis (21.5%) and angiodysplasias (14.4%). Colonic adenomatous polyps were the most frequent preneoplastic digestive lesion, with 39.6% of renal patients affected. Gastric and duodenal ulcers were found in 17.7% of cases.
Prevalence of gastrointestinal lesions.
| Gastrointestinal lesions | Total (n=255) |
|---|---|
| Esophagitis, n (%) | 8 (8.4) |
| Acute lesions of the gastric mucosa, n (%) | 21 (22.6) |
| Acute gastritis | 0 (0) |
| Bulboduodenitis | 6 (6.4) |
| Gastric and duodenal ulcer | 17 (17.7) |
| Chronic gastritis, n (%) | 39 (50.6) |
| Polyps, n (%) | 22 (19.9) |
| Gastric of fundic or hyperplastic glands | 10 (10.6) |
| Hyperplastic colonic | 12 (9.3) |
| Angiodisplasias, n (%) | 19 (14.4) |
| Ischemic colitis, n (%) | 4 (3.1) |
| Inflammatory bowel disease, n (%) | 4 (4.8) |
| Diverticulosis, n (%) | 28 (21.5) |
| Hemorrhoids, n (%) | 36 (27.5) |
| Inactive | 32 (24.4) |
| Congestive | 4 (3.1) |
| Preneoplastic lesions, n (%) | 55 (43.9) |
| Barrett's esophagus | 3 (3.2) |
| Gastric adenomatous polyps | 1 (1.1) |
| Colonic adenomatous polyps | 51 (39.6) |
| ≤2 adenomatous <1cm | 34 (26.4) |
| ≥1 adenomatous ≥1cm | 5 (3.9) |
| ≥3 adenomatous <1cm | 12 (9.3) |
| Neoplastic lesions, n (%) | 19 (15.1) |
| Gastric cancer | 1 (0.9) |
| Colorectal cancer | 18 (14.3) |
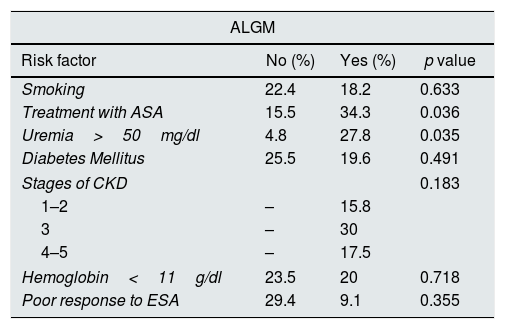
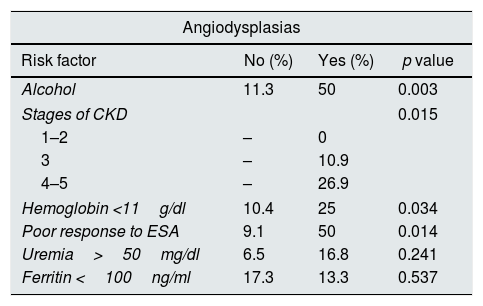
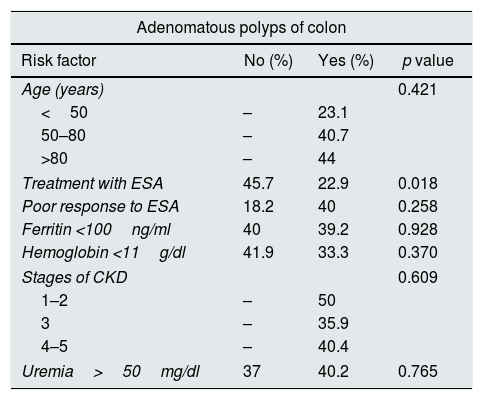
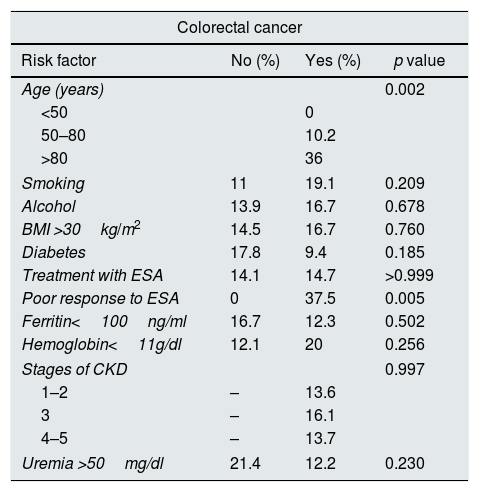
Tables 3–6 describe the risk factors associated with gastrointestinal lesions causing anemia: ALGM, angiodysplasias, preneoplastic lesions and colorectal cancer.
Risk factors associated with acute lesions of the gastric mucosa.
| ALGM | |||
|---|---|---|---|
| Risk factor | No (%) | Yes (%) | p value |
| Smoking | 22.4 | 18.2 | 0.633 |
| Treatment with ASA | 15.5 | 34.3 | 0.036 |
| Uremia>50mg/dl | 4.8 | 27.8 | 0.035 |
| Diabetes Mellitus | 25.5 | 19.6 | 0.491 |
| Stages of CKD | 0.183 | ||
| 1–2 | – | 15.8 | |
| 3 | – | 30 | |
| 4–5 | – | 17.5 | |
| Hemoglobin<11g/dl | 23.5 | 20 | 0.718 |
| Poor response to ESA | 29.4 | 9.1 | 0.355 |
ASA: acetylsalicylic acid; CKD: chronic kidney disease; ALGM: acute lesions of the gastric mucosa.
Risk factors associated with angiodysplasias.
| Angiodysplasias | |||
|---|---|---|---|
| Risk factor | No (%) | Yes (%) | p value |
| Alcohol | 11.3 | 50 | 0.003 |
| Stages of CKD | 0.015 | ||
| 1–2 | – | 0 | |
| 3 | – | 10.9 | |
| 4–5 | – | 26.9 | |
| Hemoglobin <11g/dl | 10.4 | 25 | 0.034 |
| Poor response to ESA | 9.1 | 50 | 0.014 |
| Uremia>50mg/dl | 6.5 | 16.8 | 0.241 |
| Ferritin <100ng/ml | 17.3 | 13.3 | 0.537 |
ESA: erythropoiesis stimulating agents; CKD: chronic kidney disease.
Risk factors associated with colonic preneoplastic lesions.
| Adenomatous polyps of colon | |||
|---|---|---|---|
| Risk factor | No (%) | Yes (%) | p value |
| Age (years) | 0.421 | ||
| <50 | – | 23.1 | |
| 50–80 | – | 40.7 | |
| >80 | – | 44 | |
| Treatment with ESA | 45.7 | 22.9 | 0.018 |
| Poor response to ESA | 18.2 | 40 | 0.258 |
| Ferritin <100ng/ml | 40 | 39.2 | 0.928 |
| Hemoglobin <11g/dl | 41.9 | 33.3 | 0.370 |
| Stages of CKD | 0.609 | ||
| 1–2 | – | 50 | |
| 3 | – | 35.9 | |
| 4–5 | – | 40.4 | |
| Uremia>50mg/dl | 37 | 40.2 | 0.765 |
ESA: erythropoiesis stimulating agents; CKD: chronic kidney disease.
For colorectal cancer, the risk factors were poor response to ESA (OR: 1.6 [1.1–2.4]) and age >80 years (OR: 3.52 [1.6–7.9]).
Risk factors associated with colorectal cancer.
| Colorectal cancer | |||
|---|---|---|---|
| Risk factor | No (%) | Yes (%) | p value |
| Age (years) | 0.002 | ||
| <50 | 0 | ||
| 50–80 | 10.2 | ||
| >80 | 36 | ||
| Smoking | 11 | 19.1 | 0.209 |
| Alcohol | 13.9 | 16.7 | 0.678 |
| BMI >30kg/m2 | 14.5 | 16.7 | 0.760 |
| Diabetes | 17.8 | 9.4 | 0.185 |
| Treatment with ESA | 14.1 | 14.7 | >0.999 |
| Poor response to ESA | 0 | 37.5 | 0.005 |
| Ferritin<100ng/ml | 16.7 | 12.3 | 0.502 |
| Hemoglobin<11g/dl | 12.1 | 20 | 0.256 |
| Stages of CKD | 0.997 | ||
| 1–2 | – | 13.6 | |
| 3 | – | 16.1 | |
| 4–5 | – | 13.7 | |
| Uremia >50mg/dl | 21.4 | 12.2 | 0.230 |
ESA: erythropoiesis stimulating agents; CKD: chronic kidney disease; BMI: body mass index.
The risk of suffering ALGM, in patients under treatment with acetylsalicylic acid (ASA) was 2.8 times higher (1.0–7.7) and the risk was multiplied by 7.7 in cases of uremia greater than 50mg/dl (1.2–61.2).
Alcohol consumption increased the risk of angiodysplasias by 7.8 times (2.2–27.9). The risk of suffering these injuries was 2.4 times higher (1.1–6.7) in patients with CKD stages 4–5 as compared to stage 3. Hemoglobin levels lower than 11g/dl increased the risk of having these lesions by 2.9 times (1.05–7.8) and the hyporesponsiveness to the treatment with ESA, increased the risk by 10 times (1.7–59.9).
Colonic adenomatous polyps were significantly associated to treatment with ESA (p=0.015). They were more frequent at a later age, although no statistical significance was obtained. However colorectal cancer was more frequent in older patients which was found in patients with (78.50±5.98 vs. 67.78±12.06 years; p<0.001). The treatment with ESA was associated with the presence of adenomatous polyps and the hyporesponsiveness to this drug correlated with colorectal cancer.
Although without statistical significance, esophagitis was more prevalent in patients with enolic habit (28.6% vs. 7%; p=0.10) and in smokers (14.3% vs. 5.2%; p=0.15); a 15.6% of the patients with gastric polyps were on treatment with PPI as compared to 3.3% (p=0.165). The prevalence of hemorrhoids was higher in the more advanced stages of CKD (13.6% in stages 1–2, 31.2% in stage 3 and 25.9% in stages 4–5, p=0.206). Chronic gastritis, inflammatory bowel disease and diverticulosis did not correlate with any factor.
No correlation was found between the etiology of CKD and the lesions found in this study. In no case were differences detected between gender.
The multivariate logistic regression models observed an association between esophagitis, treatment with ASA and uremia >50mg/dl, that did not reach statistical significance (p=0.78 and 0.73, respectively). An association was also observed between angiodysplasias and alcoholism and between these lesions and the stages of CKD (p=0.01 and p=0.04, respectively).
DiscussionThe present work shows a high prevalence of gastrointestinal lesions in patients with CKD and anemia.
There are no published studies reporting the prevalence of gastrointestinal lesions in CKD patiens with anemia and with positive immunochemical FOBT. Launoy et al.27 evaluated the presence of adenomatous colonic polyps and colorectal cancer in a non-renal population of 7421 individuals aged 50–74 years, of whom 434 (5.8%) had a positive immunochemical FOBT, the number of patients is greater than in the present work in which patients were not selected according to age but for criteria of anemia. In Launoy study the prevalence of adenomatous polyps was 49.5%, somewhat higher than ours (39.6%), while that of colorectal cancer was lower (6% vs. 14.3%).
There are studies in renal patients using a guaiac FOBT. The most important is the one by Bini et al.,28 who formulated the hypothesis that the predictive value of a guaiac FOBT would decrease with the degree of renal failure, depending on the presence of intestinal bleeding from trivial mucous lesions. They prospectively identified 1225 asymptomatic individuals with moderate risk of colorectal cancer, who had been referred for a colonoscopy because a positive FOBT. Clinically relevant lesions were found in 23.9% of patients with CKD stage 1, in 32.8% of patients with CKD stages 2 and 3, and 42.6% of patients with CKD stages 4 and 5. The gastrointestinal lesions increased with the degree of renal failure: adenomas of 1cm or greater (15.1, 20.1 and 22.8%), carcinomas (5.1, 10.1 and 13.2%) and vascular ectasias (1.7, 2.4 and 6.1%). Compared with patients without renal failure or CKD stage 1, the risk of having a clinically important lesion was estimated at 1.61 in subjects with CKD stages 2 and 3 and 2.33 in CKD stages 4 and 5. It was concluded that the predictive value of a positive FOBT for clinically significant lesions increased according to the degree of CKD. These findings are consistent with those of the present study, in which the ALGMs increase with the degree of uremia (27.8% vs. 4.8%, p=0.035) and the prevalence of angiodysplasias increases with the degree of renal insufficiency (0% in stages 1–2 vs. 17.6% in stages 3–5, p=0.024).
The prevalence of gastrointestinal lesions in patients from the present study is, in general, greater than that observed in pre-kidney transplant studies, such as that of Al-Mueilo et al.10 which showed: chronic gastritis 50.6% vs. 37%; ulcer 17.7 vs. 11%, bulboduodenitis 6.4 vs. 14.8%), Khedmat et al.9 (ulcer in 17.7% vs 16.1% in patients with CKD, 13.7% in hemodialysis and 13.7% in transplanted patients) and Sotoudehmanesh et al.3 (esophagitis in 5.8% vs 8.4%, ulcer in 17.7% vs 73%, angiodysplasia in 14.4% vs 4.4%, inflammatory gastric polyps in 10.6 vs. 1.5%). This finding may be due to the selection of patients under criteria of anemia. Sotoudehmanesh et al. did not correlate the lesions with age, time on dialysis, the cause of CKD, symptomatology or serum hemoglobin levels, although the most important lesions were observed more frequently in men. Lee et al.29 compared the colonoscopy of 57 candidates for renal transplantation with 60 subjects without renal failure, who underwent colonoscopy as part of a screening for colorectal cancer. Polyps were found in 37% of the transplant candidates and in 22% of the controls, which already suggested that the colorectal cancer screening guidelines should be adapted to potential candidates for kidney transplantation.
Zuckerman et al.6 showed that angiodysplasia was the most frequent cause of upper gastrointestinal bleeding, and the recurrent bleeding in patients with CKD. No comparison should be made with our work, since our patients are stable, whose reason for consultation is the revision of their CKD.
Angiodysplasias have been frequently associated with CKD. However, although the present study shows that they constitute an important group of gastrointestinal lesions related to the degree of renal function, but this lesion is not the only or the most prevalent one. Its association with hemoglobin values lower than 11g/dl and the reduced response to ESA are useful data that suggest to perform an endoscopic study prior to the prescription of ESA in patients with CKD.
It should be noted that all were out patients without symptoms that would have motivated an endoscopic study. Therefore, the finding of gastroduodenal ulcer in 17.7% and of ischemic colitis in 3.1% of the cases is striking.
Although the patients in the study were selected for the presence of anemia and a positive FOBT, they were lesions specifically related to a serum hemoglobin lower than 11g/dl or a hyporesponse to ESA; that was the case of angiodysplasias, adenomatous colonic polyps and colorectal cancer.
It has been described that patients with CKD have a higher risk of certain types of cancer due to dysfunction of the immune system, inadequate DNA repair, reduction of antioxidant defense, accumulation of carcinogens, chronic infections and inflammation.30 Most of these studies are retrospective do not offer a real number of patients diagnosed, so screening studies would be recommendable.
Wu et al.31 calculated that the risk of colorectal cancer in a population of 15,975 CKD patients not on dialysis was 0.34%.
Ito et al.32 performed a gastrointestinal tumor screening by abdominal ultrasound, FOBT, gastroscopy and colonoscopy in 178 patients on hemodialysis and peritoneal dialysis, of which 10 of them presented malignancy (one esophageal cancer, three gastric cancers, 4 colorectal cancers and two hepatocellular carcinomas). This study also confirms that the prevalence of neoplastic lesions in patients with CKD (14.3% of colorectal cancer) is much greater than that reported in non-renal patients.33
The present work includes a large number of patients with a wide age range of eGFR, it is a prospective design, with carefull collection of demographic and clinical data and the use of gastroscopy and complete colonoscopy to evaluate patients with a positive FOBT. This is the first study to determine the prevalence of gastrointestinal lesions in patients with CKD (non-dialysis) and anemia.
However, there are some limitations that should be considered. Patients with advanced CKD and dialysis have a high prevalence of mucosal inflammation and trivial lesions in the upper gastrointestinal tract, which can bleed and result in positive FOBT in the absence of clinically important lesions leading to overuse of colonoscopy. Our study highlights the importance of ruling out the gastrointestinal origin of anemia in patients with CKD, which may result in a reduction in the use of ESAs, plus the obvious benefit derived from modifying the medium and long-term prognosis of the neoplasia if that is the case. The FOBT has a low sensitivity for detecting hidden upper gastrointestinal and right colon hemorrhages, so that studies that use colonoscopy as a screening test for colorectal cancer would be necessary. The fact that this work has been performed in a single center could limit the extrapolation of the results to other populations, especially in the case of colorectal cancer, which has been shown to be associated with environmental and nutritional factors.
ConclusionsThe prevalence of gastrointestinal endoscopic lesions in patients with CKD and anemia is high, including preneoplastic and neoplastic lesions suggesting the need for its diagnosis in routine clinical practice through gastroscopy and colonoscopy. Screening would be particularly beneficial in patient at high risk such are those older than 50 years with CKD stages 3–5.
Conflicts of interestsThe authors declare that they have no conflicts of interest.
Please cite this article as: García Agudo R, Aoufi Rabih S, González Carro P, Pérez Roldán F, Proy Vega B, Arias Arias Á, et al. Lesiones gastrointestinales en pacientes con enfermedad renal crónica y anemia. Nefrologia. 2019;39:50–57.