La fibrosis nefrógena sistémica es una entidad clínica descrita inicialmente como dermopatía esclerodermiforme, en pacientes con insuficiencia renal avanzada, cuya etiopatogenia no está esclarecida. En los últimos años se ha podido comprobar la importancia del gadolinio como agente etiológico, administrado como contraste en las angiorresonancias magnéticas. El depósito de radicales libres de gadolinio sería el causante de desarrollar una fibrosis sistémica. Las guías terapéuticas aconsejan no administrar derivados con gadolinio, especialmente gadodiamida, en pacientes con filtrados glomerulares inferiores a 30 ml/min. No existe tratamiento efectivo para la enfermedad, la cual tiene muy mal pronóstico: la prevención es el arma terapéutica más efectiva por el momento, pues no existen grandes series con un número importante de enfermos tratados. Presentamos un caso con muy mala evolución, el primero descrito en España, con afectación sistémica grave, contrastada en el estudio necrópsico. Recibió repetidas exposiciones a gadolinio, principalmente gadodiamida, factor que influyó probablemente en esa mala evolución. Revisamos los aspectos más actuales de la enfermedad.
INTRODUCTION
Nephrogenic systemic fibrosis (NSF) is a new disease that has been described almost exclusively in patients with renal failure (RF) who have been exposed to radiological contrast with gadolinium. It is associated with significant morbidity and mortality and is characterised by a painful and incapacitating course. There is an acute phase after exposure to gadolinium, associated with an inflammatory response that is affected by iron metabolism, and a chronic phase characterised by predominantly progressive systemic fibrosis.
In 2000, the first cases of scleroderma-like nephrogenic fibrosing dermopathy (NFD) were described in the United States.1 It has subsequently been called NSF, especially in Europe and the United States.2,3 We will now describe the first known case in Spain.
CLINICAL CASE STUDY
A 63-year old male, whose past medical history included cutaneous allergy to bisoprolol; former smoker of two packs/day; alcohol consumption 150g/day; arterial hypertension of 13 years duration; high cholesterol; laryngeal neoplasia diagnosed 12 years previously and treated by laryngectomy without radiation treatment; simple chronic bronchitis; Helicobacter pylori-positive duodenal ulcer in 1999, the same year in which he presented with a right haemiparesis due to a left intracerebral haematoma. The patient had bilateral carotid artery disease and aortic atheromatosis with a suprarenal aortic aneurism of 3.8cm diameter.
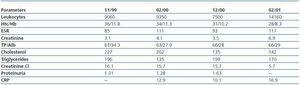
As a result of the cerebral vascular episode in 1999, he was noted to have chronic RF secondary to ischemic nephropathy due to critical stenosis of the right renal artery ostium with an atrophied right kidney, in addition to atheromatosis of the left renal artery. Between February 1999 and March 2000, the patient underwent magnetic resonance angiography (MRAs) with gadolinium four times (three with gadodiamide), two supra-aortic trunk (SAT), one aorto-renal and one thoraco-abdominal aortic angiography. Subsequently, a fifth MRA was performed due to intermittent claudication and progressive RF. The patient¿s plasma creatinine levels ranged between 2.8 and 4mg/dl with creatinine clearance below 30ml/min.
In February 2000, the patient was admitted with a toxaemia and dyspnoea. A right pleural effusion was detected and analysis of the pleural aspirate showed a lymphocytic exudate that was ADA and BK negative and cultures and cytology were also negative. Pleural biopsy revealed fibrinous pleuritis with foreign-body giant cells (cholesterol crystals) without granulomas, mycobacterial and amyloid stains were negative and there were histolopathological signs of malignancy. Thoraco-abdominal CT confirmed apart from the pleural effusion, pachypleuritis, a thoraco-abdominal periaortic mass resembling atypical retroperitoneal fibrosis and bilateral perirenal deposits.
Abdominal and perirenal subcutaneous fat biopsies were negative for amyloid and compatible with a fibroblastic reaction. Talc pleurodesis and corticoid treatment were administered with tuberculostatic treatment. Laboratory tests showed alterations in non-specific inflammatory parameters, with normal or negative immunology.
Subsequent progress continued to be very slow, with asthenia, anorexia, weight loss, nausea, loss of balance when standing and intermittent claudication. The latest SAT MRA showed a double stenosis of the right internal carotid, the larger one of 80% and on the left side of 45% stenosis. The patient underwent surgery in December 2000; the vascular surgeon observed a hardened subcutaneous tissue that was affecting the vessels, and therefore took biopsies that showed fibro-adipose tissue compatible with thick adventitia, with occasional lymphocytes. The extirpated arterial plaque showed atheromatosis, fibrosis and focal dystrophic calcification in addition to thrombosis.
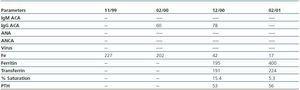
In March 2001, the patient began periodical dialysis sessions for terminal CRF. He was subsequently admitted persistent inflammatory state; Kt/V >1, Hb 10.3, and residual creatinine clearance of 5ml/min. Immunological tests, including antiphospholipid antibodies, were negative.
The nursing staff reported that it was difficult to puncture veins and stated that the skin was hardened and rough. In July 2001, the arteriovenous fistula became thrombosed (difficult repeated cannulation). A permanent tunnelled catheter was placed.
Weeks later, the toxic state continued, with the patient experiencing nausea, occasional vomiting, diarrhoea, low-grade fever with continuing elevated inflammatory markers, progressive malnutrition, leukocytosis of 24,100 and thrombocytosis of 528,000. On CXR the right pleural effusion. Persisted negative cultures. The final outcome was the death in August 2001.
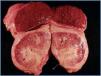
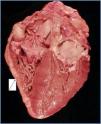
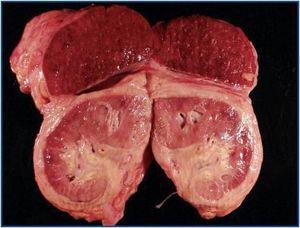
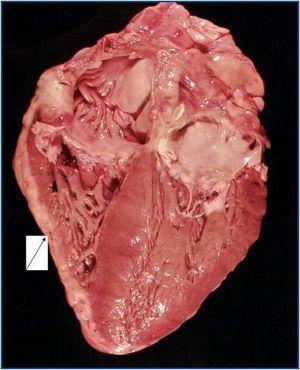
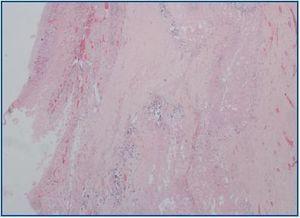
The autopsy revealed a systemic fibrosing process (figures 1- 4) affecting the lesser pelvis and retroperitoneum, encasing the kidneys, adrenal glands and pancreas, reaching the perisplenic, perihepatic and mediastinic regions. In the chest there was also encasing of the pericardium, aortic arch and major vessels and right pachypleuritis with calcified fibrosis. There was associated chronic fibrous pancreatitis, testicular atrophy, evidence of atheroembolic disease, left ventricular hypertrophy and chronic liver fibrosis, in a background of severe widespread arteriosclerosis affecting the kidneys (bilateral nephroangiosclerosis with an atrophied right kidney), brain (old cystic infarct of the left insular cortex), and colon (rectosigmoid ischaemic colitis).
DISCUSSION
The most commonly-used contrast for MR angiography is gadolinium, a contrast agent that is innocuous when renal function is normal. However, from the point of view of the nephrologist, there three important considerations, first and foremost is the risk of NSF, initially described as NFD.4 Secondly, there is a possibility that high doses of gadolinium may cause acute renal failure. And thirdly, administering gadolinium is associated laboratory abnormalities, such as pseudo-hypocalcaemia. Confronted with these possible problems, the use of gadolinium in nephrology, particularly for patients with stage III CKD and above, needs to be weighed in terms of the risk-benefit to the patient.5
Gadolinium is a rare earth element with ferromagnetic properties. More than 200 million people had been exposed to its derivatives by 2004.6 Gadolinium in its free, ionic form is toxic, is deposited in tissues, alters cellular calcium channels, neurotransmission and interferes with cellular enzymes.7 It is therefore administrated in binder form (molecule capable of binding metallic cations). The FDA in the United States has validated five derivatives for use in MRAs.7 With time, we have seen that renal clearing is very important in determining the pharmacokinetics of these molecules. Stage V CKD results in an extremely reduced elimination of these derivatives, which favours their being deposited in tissues, while in stages III and IV the risk of toxicity is less clear. Gadodiamide is the derivative that is most commonly related with this clinical profile,2 but this is probably because it is the most widely-used molecule. Exposure to these substances is not the only factor leading to the appearance of NSF. Other cofactors are needed; severe RF is the most evident. Other factors that have been considered are acidosis,8 intravenous administration of iron or erythropoietin (EPO), severe hyperphosphataemia amongst others.7 In patients with KF, iron metabolism can play an essential role in the pathogenesis of this disease: treatment with high doses of intravenous Fe, a lowered capacity for binding Fe to transferrin and haemosiderin (malnutrition, significant proteinuria), sepsis, chronic inflammatory state, etc., promote the appearance of NSF.9
In the case of EPO, it seems more likely that its relationship with NSF is consequential, not causal: patients affected with NSF have a significant inflammatory state, and therefore a high requirement for EPO. It should not be concluded that higher doses of EPO may cause NSF.10,11
Schieren G. et al. proposed hat CRP could be a good indicator of the inflammatory marker that may predict NSF, if its levels are high after administering gadolinium, since some of its derivatives increase acute-phase reactants in patients on haemodialysis.12
In a pilot study in which gadolinium was demonstrated in 4 out of 13 tissue samples from seven patients diagnosed with NSF implying a pathenogenic role for gadolinium in this clinical entity.13
The NSF initially described as a fibrosing dermopathy begins with the appearance of reddish or dark cutaneous maculae and papules inpatients with renal failure. The skin acquires a hardened texture, like orange peel and the patients complain of pain and a burning sensation all over the body except for the face. We have seen that following the cutaneous manifestations, other organs are affected, such as the musculoskeletal system, the diaphragm, myocardium, lungs, pleura, pericardium, dura mater, blood vessels etc.,3,5,9,10,14 which is exactly what occurred in our case. Some authors have described an increase in anti-phospholipid antibodies in these patients, which has not subsequently been confirmed.3 In our case they were positive but their pathogenic significance is doubtful.
The series published are based on retrospective observational studies, and so doubt can be cast on the assertions about cause (administering gadolinium) and effect (the disease), since there is doubt as to what cofactors are necessary in order for the disease to appear. Some studies informabout the appearance of NSF depending on the dose that is administered and the number of times gadolinium is re-administered.3,15
At present, NSF is a pathogenic and clinical challenge that will require a multidisciplinary approach until it is completely understood.16 Meanwhile, authors agree that administering gadolinium, and particularly gadodiamide, should be avoided in patients with a glomerular filtration rate below 30ml/min.16
The total dosage received by our patient is difficult to calculate, but it was obviously high. He received more than one gadolinium derivative, and gadodiamide on three occasions. We believe that the exposure (cause) and the effect (NSF) are clear in our case, and that they fulfil the requirements necessary to make this diagnosis.2,3,5,17,18
It has also been described that most patients who die from NSF do so from cardiovascular complications,19 as was the case with our patient. Patients with the highest mortality rate are those who have suffered a serious previous illness9 and are those with more pronounced cutaneous and cardiovascular manifestations.19 A large cumulative dose of gadodiamide, added to a high dose of erythropoietin, with a high calcium-phosphorus product would signify a higher risk of suffering from NSF; that is, existence of a serious previous illness, as proven by the presence of an acute inflammatory state.2,16
Autopsies have revealed measurable amounts of gadolinium, iron and aluminium in the heart, blood vessels and skin of these patients.19
Very recently, Agarwal R. et al.20 completed a systemised review and meta-analysis of the articles that were described and reached the conclusion that there is a clear connection between administering contrast with gadolinium and the appearance of NSF in patients with previous severe renal failure. The correlation with gadodiamide may be the most evident, but these authors state that more experience is needed to determine the risk of other gadolinium derivatives.20
Prevention is the most effective treatment for this disease. Practical guidelines do exist that warn about NSF in the use of magnetic resonance imaging with gadolinium.21 Administering gadolinium in patients with severe renal failure has to be contemplated from a risk-benefit viewpoint.17 If it is absolutely necessary, it can be administered, with a long haemodialysis session to be carried out immediately after the radiology examination with gadolinium.3 Peritoneal dialysis appears to be less effective in eliminating gadolinium derivatives.15 Improving renal function by means of a transplant can contribute to the recovery from the effects of NSF.8,22
Table 1.
Table 2.
Figure 1.
Figure 2.
Figure 3.
Figure 4.