In recent years, the concept of frailty as a “state of pre-disability” has been widely accepted by those involved in the care of the elderly. Its importance lies not only in its high prevalence – more than 25% in people over 85 years of age – but it is also considered an independent risk factor of disability, institutionalisation and mortality amongst the elderly.
The study of renal function is relevant in patients with major comorbidities. Studies have shown a significant association between chronic kidney disease and the development of adverse clinical outcomes such as heart disease, heart failure, end-stage renal disease, increased susceptibility to infections and greater functional impairment.
Frailty can be reversed, which is why a study of frailty in patients with chronic kidney disease is of particular interest. This article aims to describe the association between ageing, frailty and chronic kidney disease in light of the most recent and relevant scientific publications.
En los últimos años el concepto de fragilidad como «estado de prediscapacidad» se ha extendido de forma amplia en todos los que trabajamos en beneficio de la persona mayor. Su importancia radica no solo en su elevada prevalencia —superior al 25% en mayores de 85 años—, sino a que es considerada un factor de riesgo independiente, que confiere a los ancianos que lo presentan un riesgo elevado de discapacidad, institucionalización y mortalidad.
El estudio de la función renal es relevante en pacientes que soportan gran carga de comorbilidad, habiéndose encontrado una importante asociación entre la enfermedad renal crónica y el desarrollo de eventos clínicos adversos como la enfermedad cardiovascular, la insuficiencia cardiaca, la enfermedad renal terminal, el incremento de la susceptibilidad a infecciones y el mayor deterioro funcional.
La fragilidad puede ser una situación reversible, por lo que su estudio en el paciente con enfermedad renal crónica es de especial interés. Este artículo tiene por objeto describir las interrelaciones existentes entre envejecimiento, fragilidad y enfermedad renal crónica a la luz de la bibliografía pertinente más relevante y reciente publicada.
Chronic kidney disease (CKD) is defined in the current “Kidney Disease | Improving Global Outcomes” (KDIGO) guidelines published in January 2013, as the presence of an estimated glomerular filtration rate (EGFR) below 60ml/min/1.73m2 (for at least three months), or the existence of kidney damage observed directly in a kidney biopsy or indirectly by the presence of albuminuria, alterations in urine sediment or in imaging techniques.1,2
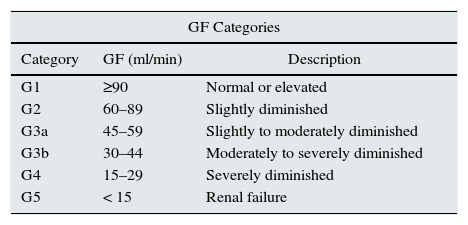
CKD classification is determined by taking into account the patient's GFR, albuminuria level and aetiology.2 The GFR levels (G1 to G5) and the albuminuria levels (A1 to A3) are shown in Table 1.
CKD classification.
| GF Categories | ||
|---|---|---|
| Category | GF (ml/min) | Description |
| G1 | ≥90 | Normal or elevated |
| G2 | 60–89 | Slightly diminished |
| G3a | 45–59 | Slightly to moderately diminished |
| G3b | 30–44 | Moderately to severely diminished |
| G4 | 15–29 | Severely diminished |
| G5 | < 15 | Renal failure |
| Albuminuria categories (isolated urine sample), mg/g | ||
|---|---|---|
| Category | Albumin/creatinine ratio | Description |
| A1 | <30 | Normal to slightly elevated |
| A2 | 30–300 | Moderately elevated |
| A3 | >300 | Very elevated |
There are now predictive equations for estimating kidney function, the formulas of which include patients’ creatinine, sex, age and weight. The abbreviated equation in the Modification of Diet in Renal Disease (MDRD) study called the Chronic Kidney Disease Epidemiology Collaboration Equation (CKD-EPI), and the Cockcroft-Gault formula are tools that are considered to be useful methods.3 Nevertheless, it should be noted that in spite of the increased use of the GFR as a screening method in clinical practice, a GFR level <60ml/min/1.73m2 does not necessarily indicate the existence of CKD, and this can lead to a false increase of this pathology, especially amongst the elderly.
In addition to having a decreased GFR, CKD also entails an inflammatory condition leading to physiological changes that affect other organs (see the section on Ageing and the mechanisms involved in chronic kidney disease). In this regard a formula has been developed that includes hematocrit, urea and gender (HUGE) and which attempts to discern whether patients with GFR <60ml/min actually have kidney disease or a GFR reduction associated with the ageing process.4 This formula has also been associated with long-term life expectancy forecasts in non-hospitalised elderly patients.5
The current prevalence of CKD in Spain is estimated to be around 9.2% of the adult population, with an overall prevalence of 6.8% in stages 3–5, but this number increases to 20.6% in patients over age 64.1,6 This increase is attributed to this population being older, due to their having greater cardiovascular risk factors, and because of earlier diagnosis.
In addition to having a higher prevalence with a forecasted increase over the coming years, CKD is also associated with adverse clinical and functional events, and with a significant cardiovascular morbidity/mortality,7–10 which justifies a considerable use of resources and a substantial increase in healthcare spending. The annual cost for treating the most advanced stages of CKD in Spain is estimated to be over €800 million.11
All this has resulted in intense attention being focused in recent years on detecting this pathology early so that its progression can be reduced. In addition to this, a decision also needs to be made whether the patient would be a candidate for replacement kidney therapy or conservative treatment, preparing the patient sufficiently in advance for therapeutic programmes such as the various types of dialysis and transplant.12–15
Ageing and the mechanisms involved in chronic kidney diseaseDescriptions have been given of how after age 30 a process occurs whereby glomerulus is replaced with fibrous tissue (glomerulosclerosis) that increases as time progresses.1,12,16,17 Meanwhile there is also an increase in mesangial tissue, with predominant obliteration of the juxtamedullary nephrons, accompanied by subendothelial deposits of hyaline tissue and collagen in the arterioles, with thickening of the intima, atrophy of the media and dysfunction of the autonomic vascular reflex. On the other hand, there are also changes in the tubules, which undergo fat tissue degeneration with enlargement of the basal membrane, with increased areas of atrophy and fibrosis.14,18,19
The anatomic changes described above lead to a decrease in the elderly patient's GFR and a decrease in effective renal plasma flow (ERPF), with a tendency towards an increase in the filtration fraction (the GFR/ERPF ratio)14,18, at the expense of a disproportionate decrease of the ERPF denominator compared to the GFR.
In approximately the third decade of life, the GFR reaches a peak of around 140ml/min/1.73m2, and from that point it begins to decrease progressively by approximately 8ml/min/1.73 m2 per decade. This is accompanied by a decrease in creatinine production, associated with a process some refer to as “senile sarcopenia”,15 which is the reason why plasma creatinine does not increase in spite of the progressive decrease in GFR.
All of these physiological changes explain the decrease in sodium reabsorption, which causes increased fractional sodium excretion in elderly patients, and a decrease in both their kidney plasma concentration and their response to stimulus, creating a state of medullary hypotonicity with a decreased urine concentration capacity.14,18
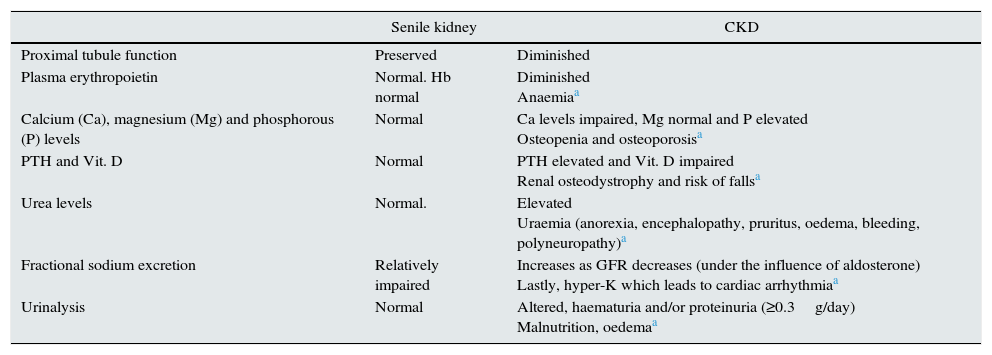
Here it is important to emphasise that even though a senile kidney will present a series of changes associated with a decrease in GFR, this is different in many aspects from the decrease in GFR that is associated with CKD (see Table 2).
Physiological aspects differentiated in CKD.
| Senile kidney | CKD | |
|---|---|---|
| Proximal tubule function | Preserved | Diminished |
| Plasma erythropoietin | Normal. Hb normal | Diminished Anaemiaa |
| Calcium (Ca), magnesium (Mg) and phosphorous (P) levels | Normal | Ca levels impaired, Mg normal and P elevated Osteopenia and osteoporosisa |
| PTH and Vit. D | Normal | PTH elevated and Vit. D impaired Renal osteodystrophy and risk of fallsa |
| Urea levels | Normal. | Elevated Uraemia (anorexia, encephalopathy, pruritus, oedema, bleeding, polyneuropathy)a |
| Fractional sodium excretion | Relatively impaired | Increases as GFR decreases (under the influence of aldosterone) Lastly, hyper-K which leads to cardiac arrhythmiaa |
| Urinalysis | Normal | Altered, haematuria and/or proteinuria (≥0.3g/day) Malnutrition, oedemaa |
GFR: glomerular filtration rate; PTH: parathyroid hormone; Vit. D: vitamin D.
In this regard, CKD per se represents a state of characteristic biochemical alterations. This is associated with a chronic inflammatory state that is predicated on the premature development of alterations in the patient's cytokine catabolism (IL-1 beta, IL-6, tumour necrosis factor-alpha [TNF-alpha]) and growth factors (insulin-like growth factor [IGF-I]).7,20 CKD patients experience a decrease in their IGF-I levels and an increase in their TNF-alpha. They are also observed to have an increase of other catabolic hormones (parathyroid hormone, glucagon, corticosteroids and angiotensin ii) in addition to deficiency or resistance to anabolic hormones such as insulin, growth hormones, testosterone and 25(OH) D3.
Recognising frailty. How does it help management?Physiological ageing has been associated with inflammation, loss of bone density, and the presence of vascular atherosclerosis. It has been acknowledged that part of this process includes a slight decline in physical and cognitive7 and metabolic functions.
When these processes occur simultaneously, physiological changes are generated that interact with each other. This may explain “unsuccessful ageing” linked to accelerated inflammation mechanisms, mineral and bone disorder, and vascular disease,7 that unleash adverse events such as falls, fractures and increased mortality.
Patients progress to a frail state following physical and biochemical alterations that cause a depletion of their physiological reserves and leave them exposed, “frail” and unable to respond to stress events appropriately.21,22
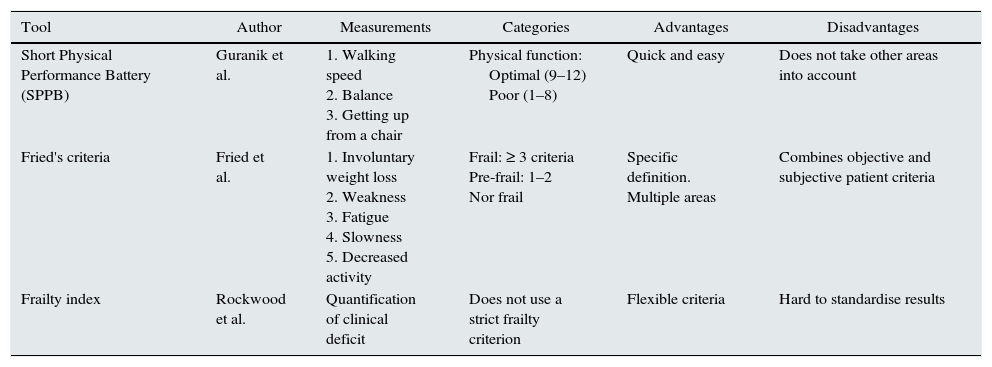
Frailty is a multidimensional syndrome,7,23 characterised by a loss in skeletal muscle mass (sarcopenia), weakness, and decreased resistance to physical exercise, and this leads to decreased activity and poor stress response. Reduced activity, in turn, makes the patient's sarcopenia and weakness worse, and this entails a tendency for them to spiral down to functional deterioration, increasing their risk of death.23,24Fried's phenotype, as defined using Fried's criteria (see Table 3), showed that a state prior to disability can be detected, thus making it deemed to be an important predictor of adverse events.25–28 Cognitive function has also been added recently.
Comparison of frailty definitions.
| Tool | Author | Measurements | Categories | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Short Physical Performance Battery (SPPB) | Guranik et al. | 1. Walking speed 2. Balance 3. Getting up from a chair | Physical function: Optimal (9–12) Poor (1–8) | Quick and easy | Does not take other areas into account |
| Fried's criteria | Fried et al. | 1. Involuntary weight loss 2. Weakness 3. Fatigue 4. Slowness 5. Decreased activity | Frail: ≥ 3 criteria Pre-frail: 1–2 Nor frail | Specific definition. Multiple areas | Combines objective and subjective patient criteria |
| Frailty index | Rockwood et al. | Quantification of clinical deficit | Does not use a strict frailty criterion | Flexible criteria | Hard to standardise results |
Data from the community has shown that there is currently a roughly 10.7% prevalence of frailty amongst patients over 65, and this figure surpasses 25% in patients over 85.8,25
In the attempt to identify which patients are more vulnerable to suffering severe adverse health events, especially disability and loss of mobility, the Comprehensive Geriatric Assessment has become more important as a basic tool for evaluating patient frailty.22,29,30 This tool provides an overall assessment of elderly patients with regard to their comprehensive condition (their clinical, functional, cognitive and psychosocial situation), with a functionality-oriented approach.
Thus a recent consensus document recommended that everyone over age 70 should be screened for frailty based on data from Frailty and Dependence in Albacete (FRADEA) study which showed that frailty entails a 5.5 times higher adjusted risk of mortality, a 2.5 times higher risk of disability, and a 2.7 times higher risk of loss of mobility.8,25
Some are now proposing that frailty might offer a perspective that could be used as a tool for better classifying patients,24,31 that would inform the medical decision-making process, prioritising the elderly patient's functionality and quality of life above all else. Table 3 shows the clinical criteria proposed for defining frailty.
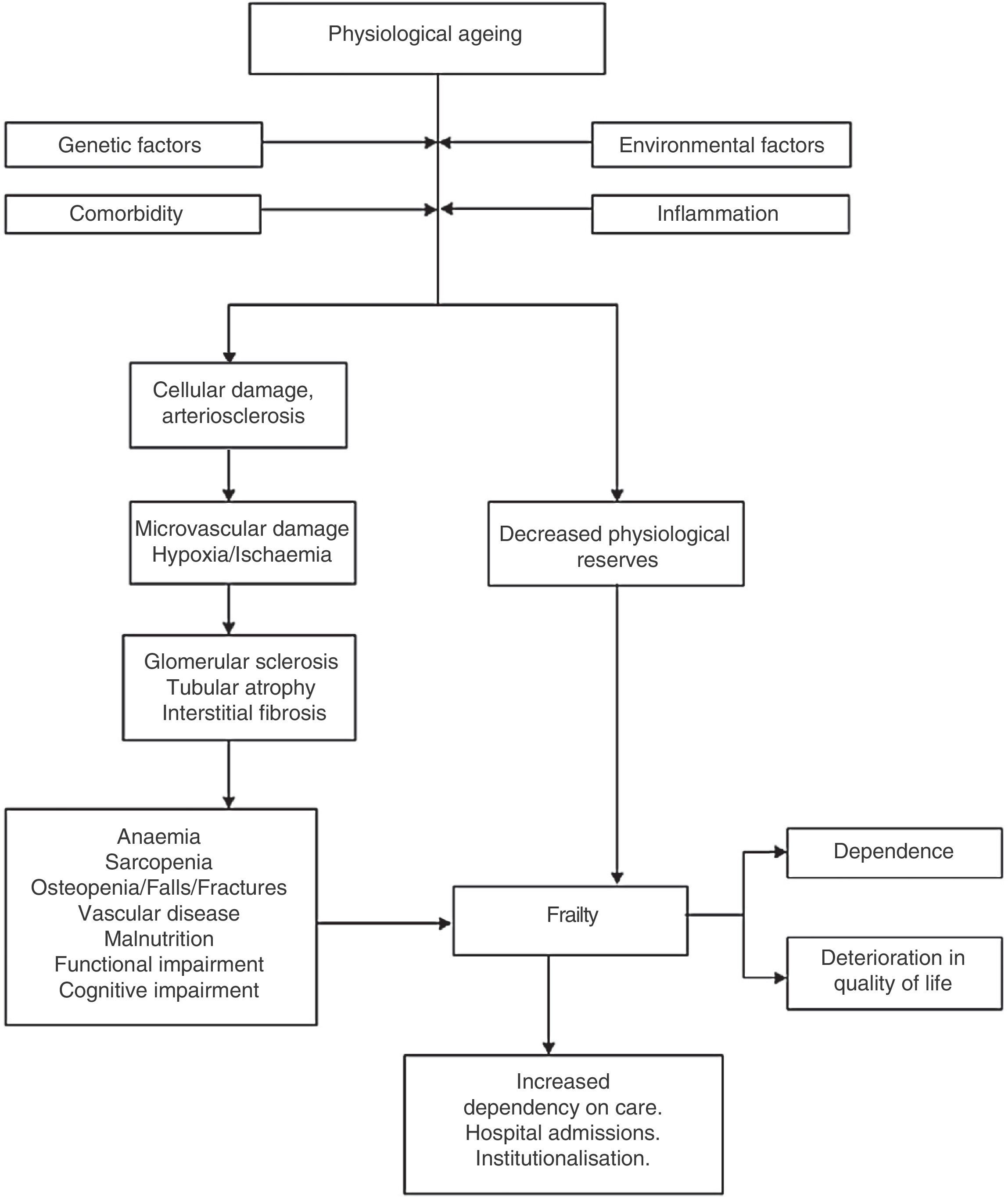
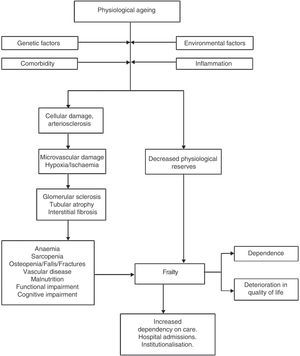
Frailty in CKDThe worst prognosis factor in elderly CKD patients has been described to be their level of dependency and comorbidity28: two conditions related to the onset of frailty. The potential frailty mechanisms in these patients are shown below (Fig. 1).
AnaemiaThis occurs following a decrease in erythropoietin (EPO) production resulting from nephron loss combined with increased resistance to EPO.7 Anaemia is a state of deficient tissue oxygenation with symptoms of low energy, impaired cognitive response and decreased physical performance,7,20 and it translates into an inability to autonomously perform the basic everyday life activities. Alterations of both EPO metabolism and iron occur with CKD, and they are associated with poor clinical evolution and deteriorating quality of life.
Inflammation and oxidative stressAs noted above, CKD is a proinflammatory state with increased C-reactive protein (CRP), IL-6 and elevated procoagulant markers, related to an increased likelihood of frailty.7,20 Some studies have observed that frail patients have higher blood levels of CRP, factor viii and D-dimer, in addition to finding an inverse relationship between patients’ physical functionality tests and their CRP and IL-6 levels.30,32 Meanwhile, due to their oxidative stress, these patients experience protein glycation (advanced glycation end products: AGEs) that is particularly accelerated in diabetic nephropathy,7 associated with deterioration of their illness.
The assumption is that if CKD worsens inflammation, then it will also worsen the patient's catabolic state, which would entail a loss of muscle mass and cachexia,25,31 and this may explain the cause of the patient's frailty and the deterioration of physical function in patients who have CKD.
ComorbidityCKD may occur simultaneously with other medical conditions, such as diabetes mellitus, chronic hypertension or malnutrition, that may play a key role in its aetiology or which may be related the physiological changes occurring in these patients.2,7,26,28 There are also other factors, such as occupational illnesses or tobacco use, that may increase the risk of developing obstructive pulmonary disease and heart failure. It is now clear that multi-morbidity is a major factor that significantly contributes to the onset of frailty in the CKD population.33
Malnutrition and sarcopeniaThere are multiple factors that link malnutrition to CKD. The main mechanisms whereby CKD contributes to the development of malnutrition are loss of appetite, dietary restrictions, a loss of nutrients (in dialysis patients) and inflammation associated with hormonal alterations and changes in catabolism.7
The loss of appetite is created by inflammation and the metabolic disorder, and this is exacerbated in the pre-dialysis stage which usually entails extremely strict dietary restrictions that aim at slowing down the progression of the CKD. This low-protein diet has been recognised as a clear factor that leads to malnutrition in these patients.
Furthermore, as noted above, biochemical changes and kidney failure boost patients’ metabolism with a significant hormonal activation. This results in a catabolic, low-energy state with progressive loss of muscle mass and strength (sarcopenia) and cachexia.25,34
Though elderly patients are recommended to add 1.0 to 1.2g/kg to their daily protein intake to maintain their physical activity, and this should be increased further if they are acutely or chronically ill or malnourished (1.2 to 1.5g/kg), this diet should nevertheless be avoided in elderly CKD patients in the pre-dialysis stage because it would hasten the advance of their kidney damage and cause them to experience uraemia secondary to retention of nitrogenous products. According to the recommendations of the PROT-AGE study on the optimal protein intake in the elderly,35 pre-dialysis CKD patients with EGF <30ml/min/1.73m2 should not ingest over 0.8g/kg per day. Nevertheless, patients undergoing dialysis may add 1.2–1.5g/kg of protein to their daily intake.
With regard to nutritional tests, the Geriatric Nutritional Risk Index (GNRI) is considered to be a useful predictor of mortality, especially for patients on haemodialysis, while the Subjective Global Assessment (SGA) is useful for pre-dialysis patients, and the MNA-SF is another useful method that has been proven to be appropriate in various patient populations.9
Cerebrovascular disease and cognitive impairmentPhysiological ageing has been associated with structural and physiological changes in the brain. The neuron loss in the cortical regions is low, but the neurons with elevated metabolism, such as neurons in the hippocampus, can be affected disproportionately by changes in synaptic function, carrier proteins and mitochondrial function. Cerebral ageing is also characterised by structural and functional changes in the microglia,25 which are the cells that are responsible for the immunity of the central nervous system. These cells are activated by brain damage and by local or systemic inflammation, and they become hyperreactive to the slightest stimulus, causing damage or even death to neurons.
Cognitive impairment is common in the various stages of CKD,36 and even though it is caused by various factors, vascular disease and specifically cerebrovascular disease clearly play a major role in the onset of CKD, especially impacting executive cognitive functions.32,36 Various studies in the literature have associated CKD patients with GFR <60, with an increased risk of ischaemic and haemorrhagic stroke.32,37
The current evidence from various observational studies supports the association between frailty, cognitive impairment and dementia.15,16,20,25
Mineral and bone disorder. Metabolism of calcium and vitamin DAlterations in bone and mineral metabolism entail an abnormal bone architecture combined with the occurrence of fractures, that in part may explain why CKD patients have decreased mobility.7 Major studies have been conducted that showed a high prevalence of hip fractures in CKD patients with GF levels under 60ml/min/1.73m2.7,15,20 Several mechanisms involving hypocalcaemia, hyperphosphataemia, hyperparathyroidism, vitamin D deficiency and metabolic acidosis have been implicated.
Various studies of the CKD population have shown that treatment with vitamin D decreased the incidence of falls and improved patients’ postural stability, thus indicating an important role in physical function and frailty.15
Other recent studies have associated the Klotho gene in the pathophysiology of CKD, finding that a decrease in the gene's expression is associated with alterations in how patients metabolise calcium (e.g., ectopic calcifications), and considering it as a possible biomarker for detecting the progression of frailty or as a future target for therapy.32,38
Functional impairmentFunctional status assessments are performed by evaluating patients’ basic and instrumental activities and their mobility. Adequate physical and cognitive capacity are needed to perform instrumental activities, and this is related to elderly patients’ independence in their activities of daily living.15,21 Aside from the factors described above (anaemia, malnutrition, cerebrovascular disease, osteoporosis, among others) that are related to impaired functional status, it should be noted that a relationship has been established between higher plasma creatinine levels and limited physical activity, with the lowest GF levels associated with dependency for at least 2 of the activities of daily living.21
CKD has also been demonstrated to have predictive value with regard to mobility limits and impaired functionality15,23,39: conditions that are induced by the illness itself and which are more transcendent than the illness itself per se.
Lastly it should be noted that these multi-factor changes that present in CKD patients indicate that their treatment must be multi-disciplinary, in that all the potential frailty mechanisms must be involved. Effective strategies need to be found that will improve these patients’ quality of life and their prognosis.
Conclusions and recommendations- 1.
Recognising and characterising frailty syndrome based on functional anthropometric dimensions instead of strictly biochemical/biological dimensions represents a major conceptual advance with practical repercussions for the clinical practices employed to manage ageing.
- 2.
It is clearly associated with a wide range of biological alterations (vascular sclerosis, a proinflammatory condition, oxidative stress, and others), the most notable of which is CKD, whose presence in frailty syndrome helped contribute to its genesis and above all to its maintenance and perpetuation via CKD-associated disorders such as anaemia, mineral and bone alterations, inflammation and malnutrition.
- 3.
Recognising the existence of frailty and assessing it with the proper measurements should be part of the clinical care for CKD in the elderly. At the same time, an awareness of the significance of CKD and the biological disorders it entails should be a key part of the clinical arsenal of general practitioners and geriatric care specialists who deal with the syndrome or condition of frailty.
- 4.
The therapeutic success/failure of the steps that are taken for CKD-related disorders in elderly patients should be gauged by considering how they will affect the patient's frailty.
The authors declare that there are no conflicts of interest.
Please cite this article as: Portilla Franco ME, Molina FT, Gregorio PG. La fragilidad en el anciano con enfermedad renal crónica. Nefrología. 2016;36:609–615.