Fibronectin glomerulopathy (FGP) was first recognized through direct staining in 1992 by Mazzucco et al.1 It was regarded as a rare autosomal dominant disease that tends to happen in various age groups and affects both sexes. The clinical features of this disease were proteinuria, typically nephrotic range, microscopic hematuria, hypertension and a slow decline in renal function.1–11 However, some patients progress to end-stage renal disease (ESRD) and dialysis has been reported.5,12–14 Herein we reported a case of FGP that was diagnosed based on the classic ultrastructural features described above. This case was unique in that FGP presents in a male who was 88 years of age and has no known family history of FGP.
An 88-year-old gentleman was admitted with anuria requiring dialysis for uremic encephalopathy and volume overload. He had an extensive past medical history including chronic renal failure (baseline Cr 200–240μmol/L) one month ago, hypertension, obstructive sleep apnea, mild cognitive impairment, query previous congestive heart failure, diastolic dysfunction, COPD, macular degeneration, benign prostatic hypertrophy and an anemia that was being investigated as an outpatient. Baseline level of proteinuria was unknown. There was no family history of kidney disease.
Relevant initial blood work included a serum creatinine of 1148μmol/L, urea 82.2mmol/L and potassium 5.7mmol/L. During his stay in hospital, a 24-h urine collection was done and only 100mL of urine were collected with 100mg of protein and a protein excretion of 1g/L. The other results of blood examination are as follows: a normal ESR, C3 and C4 values, negative ANA, c-ANCA, p-ANCA, anti-GBM antibody, hepatitis B and C serology, renal ultrasound showed normal size of the kidney. The extranuclear antigen screen was negative except RNP. Immunofixation revealed a serum IgG M-protein with kappa light chain specificity. Bone marrow studies were unremarkable.
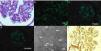
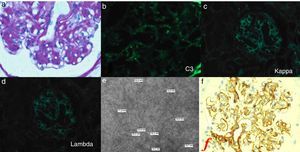
A renal biopsy was performed and light microscopy showed a lobular appearance with cellular mesangial nodules that were expanded by matrix. There was also prominent arteriolar fibrointimal hyperplasia and arteriolar hyalinosis. Congo red staining was negative for amyloid. Immunostaining was 2+ for C3 in a granular pattern in the glomerular capillary walls, however staining was negative for IgA, IgG, IgM, kappa, lambda, albumin, fibrin and C1. Electron microscopy showed prominent expansion of the mesangial and subendothelial regions with numerous randomly arranged fibrils seen under high power lens, measuring 10.7–16.8nm in diameter. Polyclonal rabbit antibody (DAKO, A0245) was used in staining of fibronectin and there was positivity in the mesangium, mesangial nodules and capillary walls (Fig. 1).
The patient received regular hemodialysis and other supportive treatment including anti-hypertension, improving anemia, and preventing renal osteopathy. Unfortunately, this patient died of heart attack half a year later.
To our knowledge, most cases of FGP have presented and been diagnosed in patients under 65-year-old. As this patient was diagnosed with FGP at the age of 88, we reviewed the cases reported in literatures and attempted to explain how this patient's disease course fits within our understanding of FGP.
A PubMed search using the terms “fibronectin glomerulopathy” yielded 16 articles reports in English of patients with biopsy and fibronectin staining proven FGP. This allowed us to compare the disease features of 64 patients, including the patient described in this paper (Table 1). These patients received renal biopsy at the age of 3–88, which was a wide range. The majority of patients had proteinuria for more than 5 years before being diagnosed, which supported the idea that the onset of disease occurs at younger ages. Most of the reported cases were patients in European countries, and there were several cases reported in Asia in recent years.
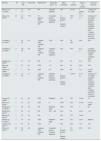
Reported cases of fibronectin glomerulopathy.
| Reporter | No. | Age at Bx | Femal:male | Hypertension | Proteinuria (g/24h) | Micro-hematuria | Cr (μmol/l) | Time of last outcome | Last renal outcome |
|---|---|---|---|---|---|---|---|---|---|
| Mazzucco et al. (1992)1 | 2 | 14, 33 | 2:0 | NO | 2 patients: nephrotic | N/A | 53,141 | 1 patient: 4 yr | 1 patient: Cr was stable |
| Strom et al. (1995)2 | 23 | 14–59 | 7:16 | 10 patients: YES 8 patient: NO | 21 patients: positive (9 patients: nephritic) | 12 patients: positive 7 patients: negative | 45.8–137 | 1–15 yr | 9 patients: Cr was stable 4 patients: dialysis 4 patients: transplant 4 patients: dead 1 patient: normal Cr, renal cell carcinoma |
| Assmann et al. (1995)12 | 2 | 19, 46 | 0:2 | 1 patient: YES 1 patient: NO | 2.8,6 | N/A | 76, 100 | N/A | N/A |
| Gemperle et al. (1996)13 | 9 | 20–46 | 3:6 | 8 patients: YES 1 patient: NO | 7 patients: positive (1 patient: nephrotic) | N/A | N/A | 6–21 yr | 2 patients: hemodalysis 2 patients: CAPD 3 patients: dead |
| Fujigaki et al. (1997)3 | 1 | 47 | 1:0 | NO | 1.4 | NO | 53 | 9 yr | 0.82 |
| Sato et al. (1998)15 | 1 | 23 | 0:1 | YES | 1–2 | NO | 80 | N/A | N/A |
| Niimi et al. (2002)4 | 1 | 3 | 0:1 | YES | >8 | YES | 80 | 7 yr | Cr was stable |
| Castelletti et al. (2008)5 | 8 | 12–59 | 1:7 | 3 patients: YES 4 patient: NO | 6 patients: positive (4 patient: nephrotic) | 2 patients: positive 1 patients: negative | <194.5 | 2–23 yr | 4 patients: Cr was stable 2 patients: dialysis 1 patients: transplant 1 patients: dead 1 patient: normal Cr, renal cell carcinoma |
| Yong et al. (2009)6 | 1 | 41 | 0:1 | YES | Nephrotic | N/A | 150 | 10 mo | 1.2 |
| Brcic et al. (2010)7 | 1 | 34 | 0:1 | YES | 6 | YES | N/A | 21 mo | Cr was stable |
| Nadamuni et al. (2012)8 | 1 | 50 | 1:0 | NO | Nephrotic range | YES | 70.7 | 1 | 0.8 |
| Otsuka et al. (2012)9 | 1 | 49 | 0:1 | NO | <0.5g/d | N/A | 92 | 9 mo | 1.54 |
| Ertoy et al. (2013)10 | 1 | 24 | 0:1 | NO | 1 | NO | 72.5 | 16 mo | |
| Ishimoto et al. (2013)14 | 1 | 78 | 1:0 | YES | Nephrotic range | N/A | 101 | 2 mo | Dialysis |
| Yoshino et al. (2013)11 | 1 | 67 | M | YES | 6 | N/A | 88.4 | 7 yr | 3.54 |
| Chen et al. (2015)16 | 10 | 19–46 | 4:6 | 4 patients: YES 6 patient: NO | 10 patients: positive (2 patient: nephrotic) | 4 patients: positive 6 patients: negative | 47–312 | N/A | N/A |
HTN: hypertension.
Bx: biopsy.
Note: If a particular piece of information was not available, the space is filled with “N/A”.
FGP is understood to present with nephrotic range proteinuria and we found 23 (35.9%) of the 64 cases that reported proteinuria were nephrotic range. 21(32.8%) patients had microhematuria at the time of biopsy. Slightly more than half of the cases had hypertension. There was no association between degree of proteinuria, microhematruia and hypertension. The degrees of renal impairment at the time of biopsy were variable. Most of them had slow decline in renal function. 22 (34.3%) patients’ serum creatinine was stable, and 24 (37.5%) patients progressed to ESRD or dead. What appears different was that our patient had severe renal impairment at the time of his diagnosis.
In general, the timing of disease seems to be the distinguishing feature of our patient. Our patient had a history of chronic kidney disease on the basis of hypertension, however he came to hospital with ESRD requiring dialysis and a renal biopsy led to the discovery of FGP. With the usual disease course of FGP being slowly progressive with proteinuria of several years’ duration, it is possible that our patient had FGP and hypertensive kidney disease for many years and that a third insult may have precipitated ESRD. In his case it may have been the concomitant pneumonia.
In conclusion, FGP is an autosomal dominant disease that has an indolent disease course after a typical presentation in adulthood. Here we reported a case of FGP diagnosed in an 88-year-old gentleman at the time of his progression to ESRD. This may suggest that he had an exceptionally long indolent course if the onset of disease was during middle age, or perhaps he had an atypically late onset of disease. Further studies are needed to characterize the disease course in FGP as well as the tendency of FGP to ESRD.
Yours sincerely,