We report the case of a 32-year-old male diagnosed with TSC2/PKD1 contiguous gene syndrome, presenting with tuberous sclerosis complex (TSC) and autosomal dominant polycystic kidney disease simultaneously. He progressed to end-stage renal disease and received a kidney transplant at the age of 12. The native kidneys presented angiomyolipomas (AML), which are common benign tumours in patients with TSC. Seventeen years after transplantation, he presented with abdominal pain, anaemia and a retroperitoneal haematoma, the latter caused by renal AML bleeding. Selective embolisation was performed. Our patient could have benefited from the administration of mTOR inhibitors at transplant. This therapy is immunosuppressive and reduces the size of benign tumours in TSC as well as the risk of rupture and bleeding. This patient did not receive mTOR inhibitors at the time of the transplant because the relationship between mTOR inhibitors and TSC was unknown at that time. This case confirms the persistent risk of renal AML bleeding for both transplanted patients and patients on dialysis. As a result, we would recommend routine check-ups of native kidneys and nephrectomy assessment.
Presentamos el caso de un varón de 32 años, con síndrome de genes contiguos TSC2/PKD1, que le ocasiona complejo esclerosis tuberosa (CET) y poliquistosis renal autosómica dominante simultáneamente. Evolucionó a enfermedad renal terminal y se realizó trasplante renal a los 12 años. Los riñones presentaban angiomiolipomas (AML), que son tumores benignos frecuentes en pacientes con CET. A los 17 años postrasplante, presentó un cuadro de dolor abdominal, anemización y hematoma retroperitoneal. Dicho hematoma se produjo por el sangrado de los AML. Como tratamiento se realizó embolización selectiva. Nuestro paciente podría haberse beneficiado en el momento del trasplante renal del tratamiento con inhibidores de mTOR. Este fármaco actúa como inmunosupresor y reductor tumoral en la CET, al disminuir el riesgo de rotura y hemorragia. En este paciente no se administró porque cuando se trasplantó no se conocía la relación de los inhibidores de mTOR con la CET. Este caso confirma que, a pesar de tratarse de pacientes trasplantados o en diálisis, el riesgo de sangrado por los AML persiste, por lo cual se propone realizar controles periódicos de los riñones propios y valorar la nefrectomía.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary renal disorder, with an incidence between 1/400 and 1000.1 It causes 10% of the cases of end stage renal disease (ESRD) in our country.2 ADPKD is a genetically heterogenous; two of genes that cause the disease are PKD1 and PKD2.3,4 The PKD1 gene is located in the chromosome 16p13, adjacent to TSC2, one of the genes responsible for the tuberous sclerosis complex (TSC).5,6 This disease is caused by mutations in that gene or by mutations in the TSC1 gene. A deletion involving the PKD1 and TSC2 genes, results in a “contiguous gene syndrome” (CGS).7 The syndrome is characterised by severe clinical signs of both ADPKD and TSC. The presence of large kidneys with multiple cysts at birth is similar to that observed in advanced stages of ADPKD, causing ESRD in the second or third decades of life. Actually there is only one case reported of a patient with both diseases caused by independent mutations of the PKD1 and TSC2 genes.8
The TSC1 gene encodes for the protein hamartin and the TSC2 gene for the protein tuberin so the complex hamartin/tuberin complex is formed.9 This complex is involved in mTOR cell signalling, the target of rapamycin which acts on the Rheb/mTOR/p70S6K signalling pathway. This cell signalling pathway is involved in cell growth, cell cycle and apoptosis. Mutations in this complex cause a disregulation of the mTOR cascade resulting in increased cell proliferation. In addition, polycystin 1, a protein encoded by the PKD1 gene, interacts with the hamartin/tuberin complex and suppresses the activity of mTOR.10
Tuberous sclerosis is an autosomal dominant systemic disorder with an elevated penetrance and a prevalence of 1/6000.11 Clinical manifestations include cutaneous lesions (angiofibromas, ungual fibromas, hypomelanic spots), angiomyolipomas (AML) in kidneys, pulmonary lymphangioleiomyomatosis, cardiac rhabdomyomas and neurological lesions such as tubers and astrocytomas. Its clinical presentation is highly variable: from minimal or unnoticeable symptoms to severe neurological involvement.11
Renal AMLs associated with TSC are often multiple and bilateral, with an incidence of 55–75% depending on age.12 They are formed by immature smooth muscle cells, fat and vascular tissue. The main threat is their risk of rupture and bleeding, which may be life threatening. AMLs are diagnosed by ultrasound, but it is most common to use computed tomography (CT) or magnetic resonance imaging (MRI), which allows better delineation of lesions. The diagnosis is difficult due to the low fat component, MRI is more useful.
Classically the therapeutic options for the treatment of AML embolisation, partial nephrectomies or radical nephrectomy in case of AML bleeding. Embolisation may cause post-embolisation syndrome13,14 which results from an inflammatory response to isquemia induced necrosis. These intervention reduce the functioning renal mass, which in this chronic and multifocal disease is very deleterious.
The mTOR inhibitors have been approved as a first-line therapy for AMLs in TSC.15 These drugs have shown some efficacy in reducing the progressive increase in kidney volume of ADPKD, but they did not show an effect on renal function.16,17
Following renal transplantation, ADPKD kidneys typically reduce their volume and present relatively few complications.18 In a similar way, it is assumed that, after transplantation, the decrease in renal flow causes a reduction of AML size which should diminish the risk of bleeding, however this remains to be demonstrated.
The present case does not support the above hypothesis; the risk of bleeding persists for many years after renal replacement therapy is commenced.
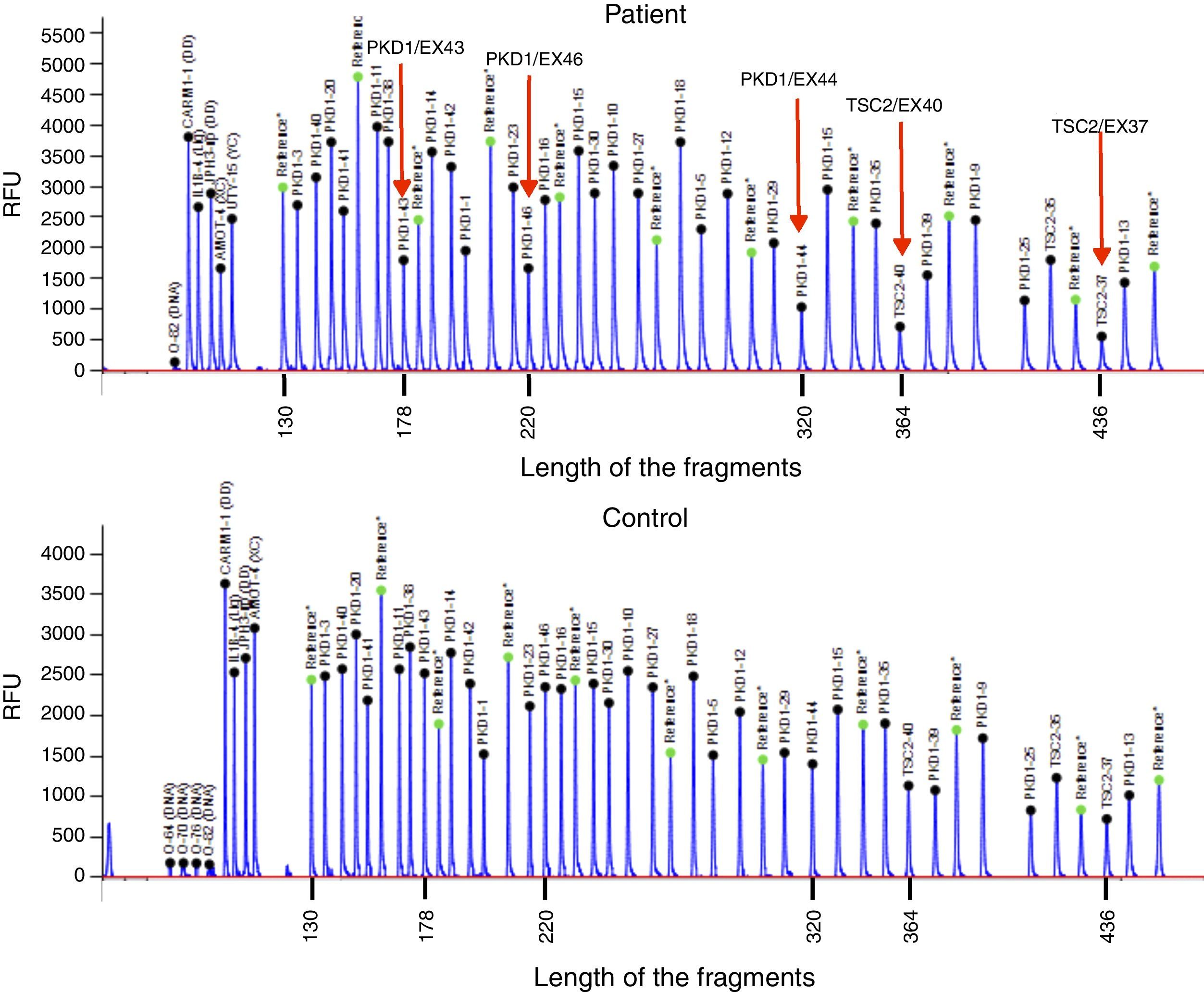
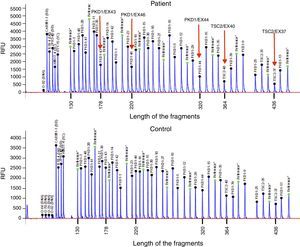
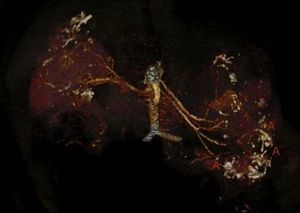
Case reportThe patient is a 32-year-old male with a history of CGS with a TSC2/PKD1 deletion. He was diagnosed at 8 months of age in the context of neurocognitive retardation. The genetic evaluation included the analysis of deletions/duplications of one or various exons by the multiplex-ligation-dependent probe amplification technique. The patient was a carrier of a de novo deletion causing the CGS. This deletion eliminates exons 43 to 46 of the PKD1 gene and exons 37–42 of the TSC2 gene (Fig. 1). The patient is a sporadic case as parents did not have the disease. Clinically, he had partial-generalised convulsive seizure episodes due to the presence of cortical tubers, as well as severe mental retardation. At birth, he had bilateral abdominal masses identified as polycystic kidneys. At age 11, a progressive deterioration of renal function was observed and renal replacement therapy using peritoneal dialysis was started 4 years later. During the following year, he received a transplant from a deceased kidney donor and immunosuppressive treatment included prednisone, cyclosporin and azathioprine. An excision of a Köenen tumour on the right foot was performed at age 26. The patients suffered from several episodes of recurrent community-acquired pneumonias.
Deletion of TSC2/PKD1 genes. Diagram of the results of deletion/duplication testing using the multiplex-ligation-dependent probe amplification technique in a patient with CGS (upper panel) and a control individual (lower panel). The red arrows indicate the deletion of exons 43–46 of the PKD1 gene (GenBank reference sequences: NM_001009944.2; NP_001009944.2): c.11713-?_12909+?del; p.(Ser1555fs) and exons 37–42 of the TSC2 gene (NM_000548; NP_000539): c.4663-?_5421+?del; p.(Val3905fs). Classified according to Human Genome Variation Society (http://www.hgvs.org/mutnomen/).
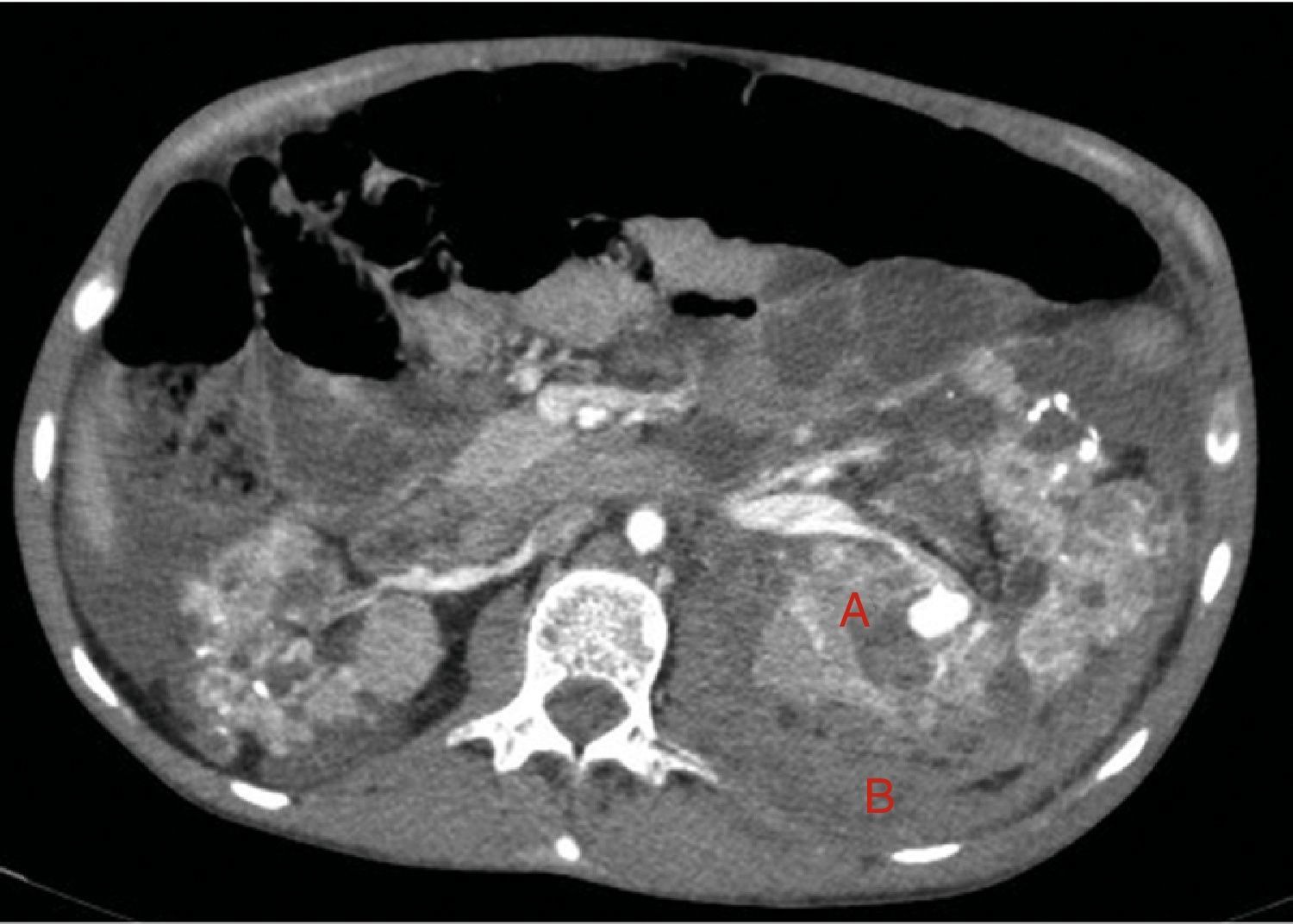
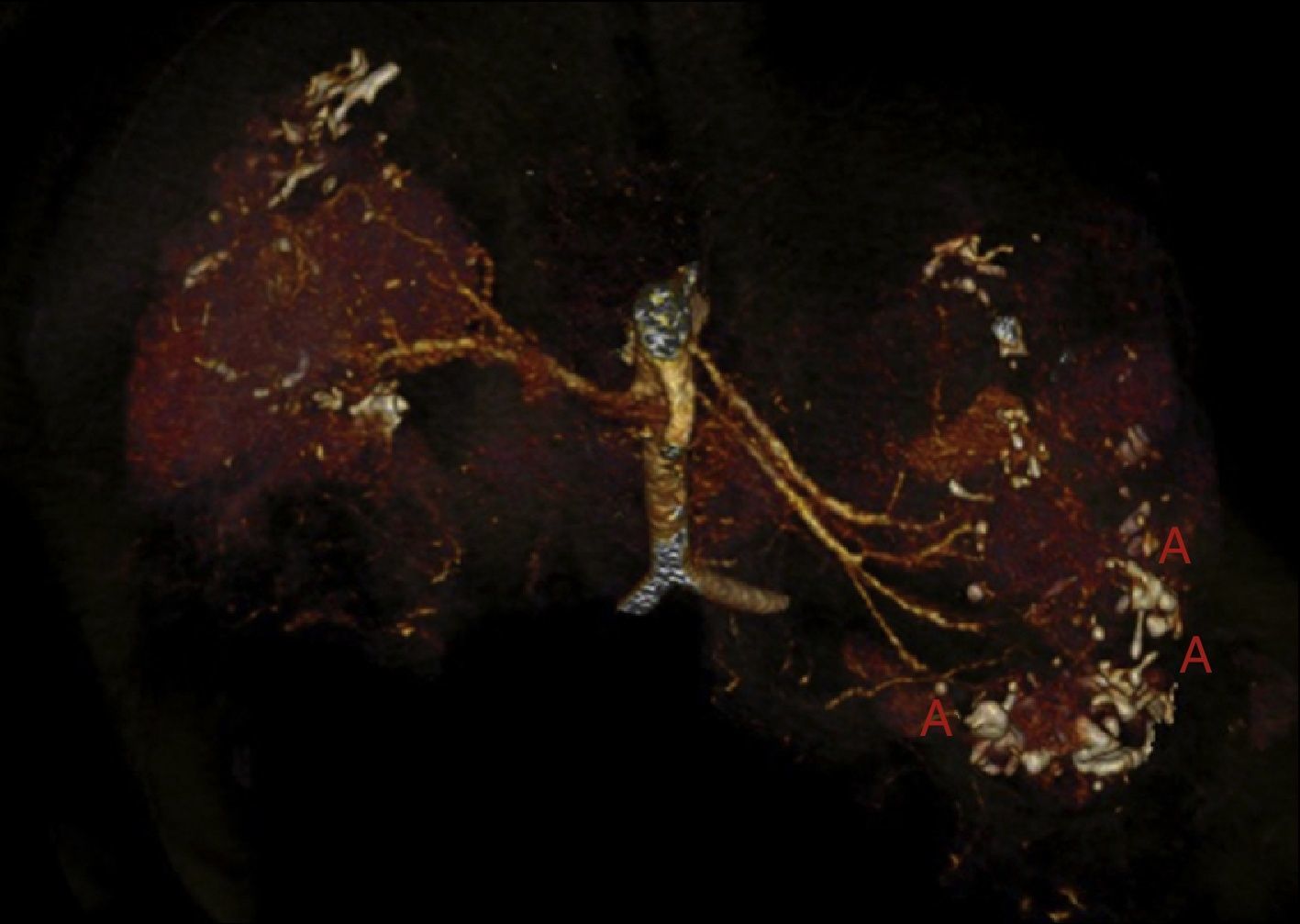
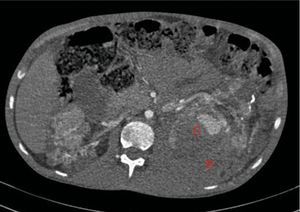
At age 32, he came to the emergency room for 3 days of abdominal pain, distension and liquid depositions with no pathological products. The clinical symptoms were controlled but asthenia prevailed. The physical exam revealed no fever, haemodynamically stability, diffuse abdominal pain upon gentle palpation but without rebound. Lab work showed haemoglobin 71g/l (normal range: 140–180g/l), haematocrit 0.22l/l (normal range: 0.40–0.52) without signs of haemolysis or sepsis. Renal function remained stable with metabolic acidosis (creatinine 232μmol/l, glomerular filtration [CKD-EPI] 30ml/min/1.73m2, serum bicarbonate 16mEquiv./l). Abdominal ultrasound showed free perihepatic fluid, abdominal CT was requested which revealed a left retroperitoneal haematoma (60mm×62mm×52mm) and multiple AML with bilateral microaneurisms; the largest was 10mm diameter at the level of the left native kidney (Figs. 2–4). The patient received two packed red blood cells, prophylachtic antibiotics, and was maintained monitored under observation. The patient progressed satisfactorily and he was discharged from hospital.
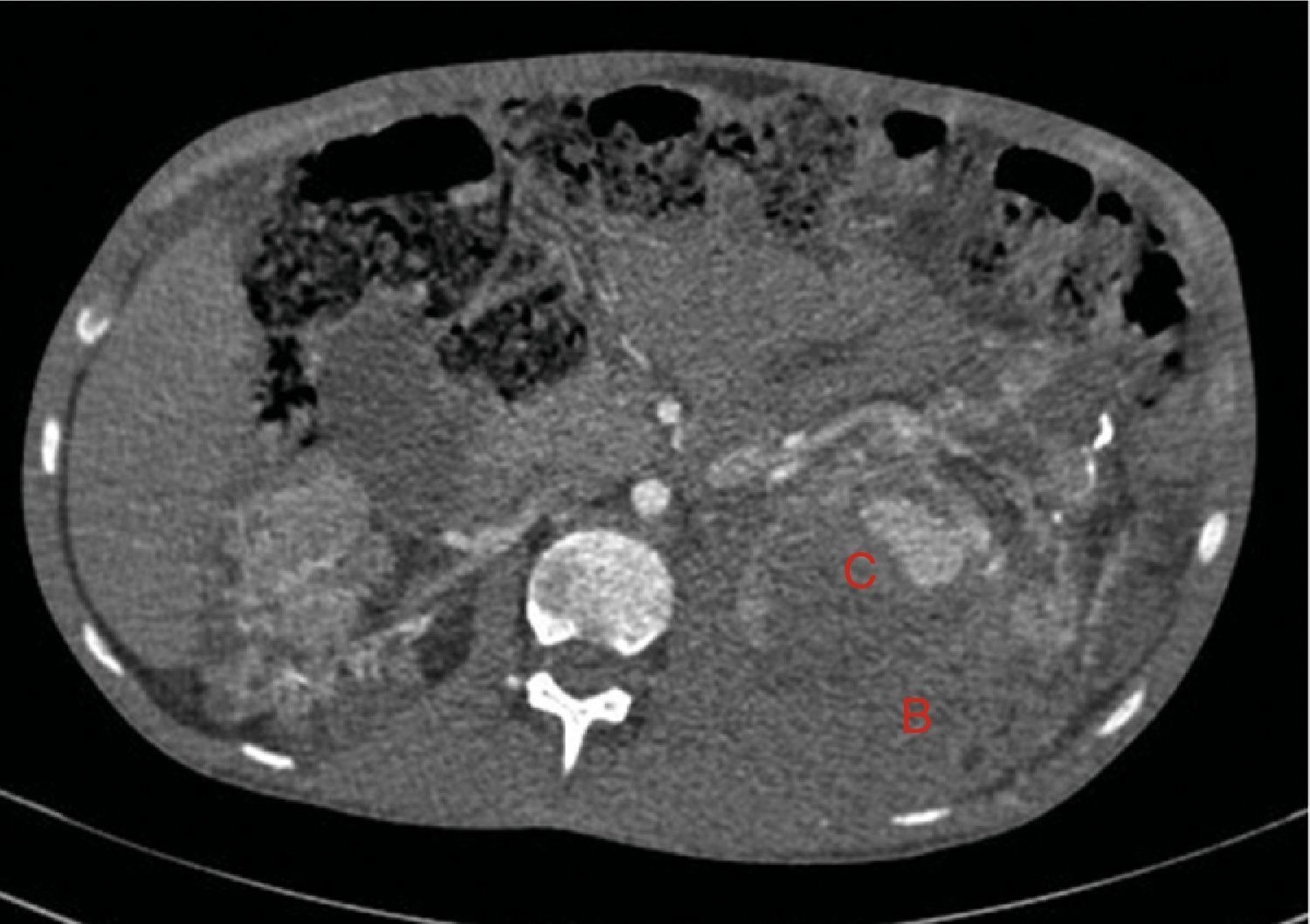
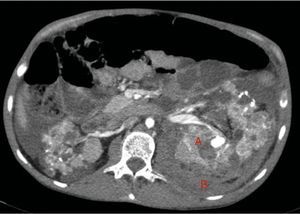
Native kidneys with multiple cysts, some with calcified walls. Lesions suggestive of angiomyolipomas with multiple bilateral aneurisms, the largest is 10mm of diameter located in the upper third of the left kidney (A). Left retroperitoneal bleeding (B) that spreads out through the posterior pararenal space.
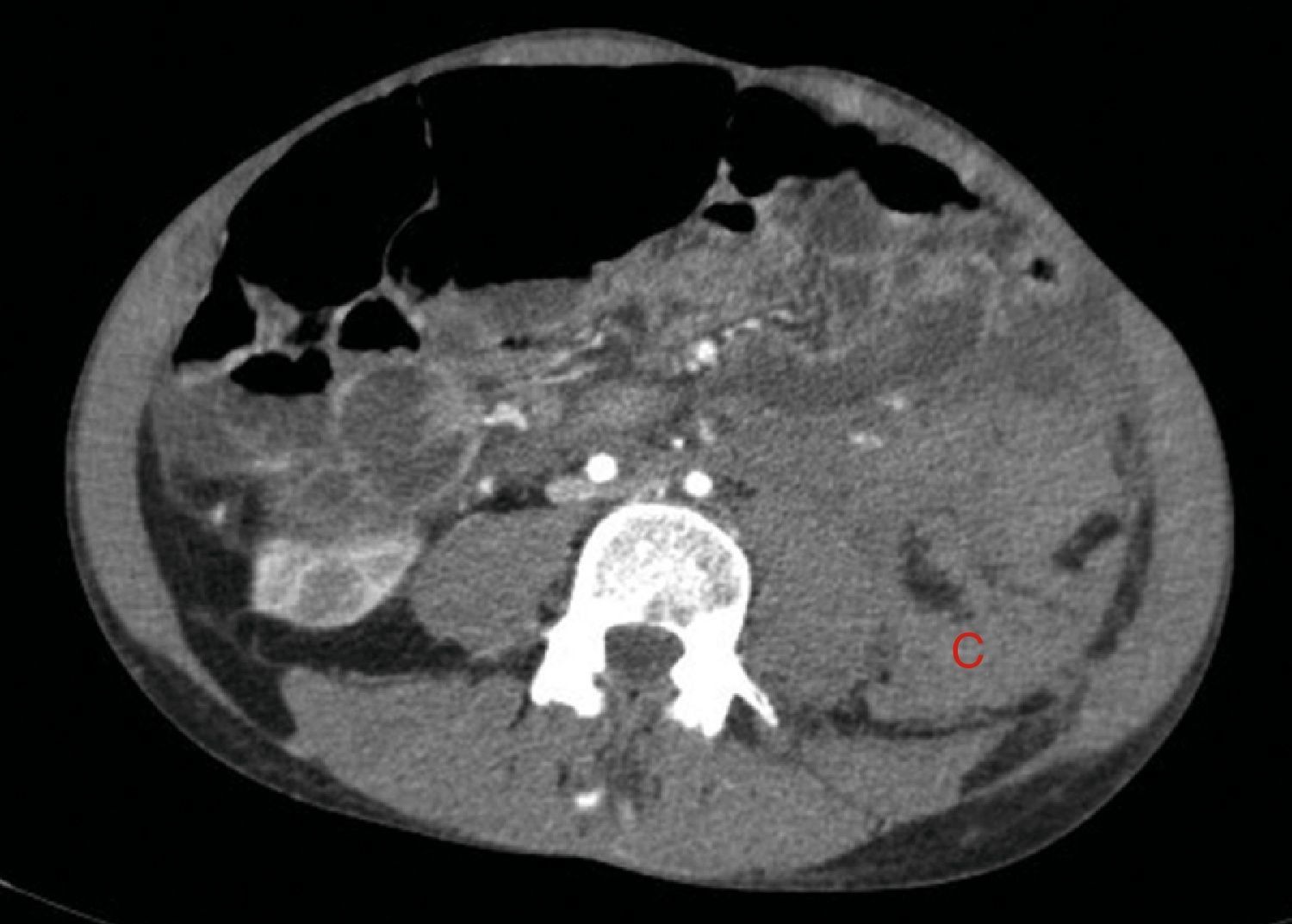
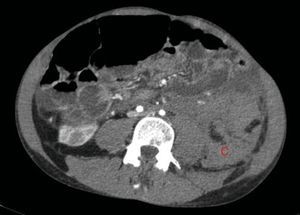
The bleeding described in Fig. 2 is the cause of a voluminous haematoma distal to the inferior renal pole (C).
Fifteen days later he consulted for low grade fever and abdominal pain, with no symptoms of bacteraemia or any source of infection. The laboratory tests showed stability in haemoglobin levels, without white count elevation, but the RCP was 174 (normal range <10), the serum creatinine was 300μmol/l and glomerular filtration rate (CKD-EPI) 22ml/min/1.73m2. Blood and urine cultures were negative. Chest and abdominal X-ray were normal. Forty eight hours later the haemoglobin dropped to 69g/l, with haematocrit of 0.21l/l and the abdominal CT scan showed an increase in left retroperitoneal haematoma (80mm×170mm×100mm) and an increase in the size of the pseudoaneurysm of the upper third of the left kidney as compared with the previous study (Fig. 5). Selective embolisation of the left renal artery was performed without complications. Two packed red blood cells were transfused. As prophylaxis for the postembolisation syndrome, corticoids were increased to 0.5mg/kg analgesics were prescribed and third generation cephalosporin was initiated. The patient did well and he was discharged 5 days later.
DiscussionIt is unusual for an individual to have mutations in 2 different genes and, therefore, have both diseases. The exception to this rarity is the CGS as in the case of the deletion of genes TSC2/PKD1.7
About 5% of the patients with TSC have ADPKD at the same time, because of a deletion involving the TSC2 and PKD111 genes. The first cause of death in TSC are neurological complications followed by renal complications.11 Patients with TSC may have renal cysts, but the most common manifestation and cause of increased morbidity are AMLs. These benign tumours may bleed, and the risk of bleeding increases with size. It is very important to diagnose the presence of microaneurisms in the AML because, if present, AMLs have a high risk of rupture.12
Rapamycin and everolimus are immunosuppressant drugs that have the ability to block mTOR activity which produces an antiproliferative effect, inhibiting T cell activity and decreasing the risk of angiogenesis by reducing the levels of vascular endothelial growth factor. Therefore, the size of the AML, fibromas, astrocytomas, and, most likely, the frequency of epileptic seizures are reduced. Cabrera et al. reported that, in 17 patients with TSC treated with rapamycin, 100% had a greater than 50% decrease in the size of the AML at one year of treatment. This decrease in size remained stable during the 1st and 2nd year of treatment.19,20 The randomised, multicentre study EXIST-2, with a sample of more than 118 participants, demonstrated a high efficacy of everolimus in decreasing AML volume, and the absence of bleeding in treated patients.15,21 Other studies also support the benefit of mTOR inhibitors in the treatment of AML.22,23 These suggest that mTOR inhibitors are the drug of choice in transplant patients with TSC.
With this information in mind, we believe that our patient could have benefited from treatment with mTOR inhibitors after his kidney transplant. This drug acts as an immunosuppressant and tumour reducer in TSC by decreasing the risk of rupture and severe haemorrhage. The tolerance of mTOR inhibitors is not always excellent, and they have to be withdrawn in some patients due to undesirable effects and poor tolerance. It is also known that its introduction with diminished glomerular filtrates is associated with functional impairment of the renal graft, especially when there is proteinuria greater than half a gram. In case, it was not administered because the relationship of mTOR inhibitors with TSC was not known when the patient was transplanted. By the time that a beneficial effect of mTOR was known, the glomerular filtration was less than 30ml/min/173m2 and certain degree of proteinuria was present due to chronic graft dysfunction, so it was not considered appropriate to use mTOR inhibitors. The possibility that, 17 years after the transplant, the kidneys themselves had sufficient renal perfusion to cause bleeding was not seriously considered. This case confirms that the risk of bleeding associated with AML persists, and therefore the use of mTOR inhibitors in a renal transplant patient with AML from TSC may be help to maintain graft function and reduce the size of the AML, lowering the risk of complications. It should be noted that our patient's right kidney also had multiple AMLs, the largest of which was 5cm. However, since he was a institutionalised patient with a limited quality of life, we tried not to be very aggressive with the treatment and limit ourselves to the control of the acute bleeding. Nonetheless, the embolisation of the right kidney still needs to be performed. In this case, renal embolisation was chosen because it is a less invasive procedure and with less risk of complications than a surgical procedure such as nephrectomy.
Based on our experience, we recommend monitoring these patients by angio-CT or MRI to detect AMLs with a risk of bleeding and to evaluate embolisation or prophylactic nephrectomy when the use of mTOR inhibitors is not suitable. The present case of TSC confirms that embolisation is an acceptable and safe technique to control AMLs bleeding or those with serious risk of bleeding that cannot receive mTOR inhibitors.
In conclusion, even in patients with TSC who receive renal replacement therapy, periodic monitoring of their own kidneys should not be discontinued. If an AML larger than 3cm is observed, it is advisable, if feasible, the use of mTOR inhibitors in transplant patients, or either renal embolisation or nephrectomy in dialysis or transplanted patients.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Furlano M, Barreiro Y, Martí T, Facundo C, Ruiz-García C, DaSilva I, et al. Sangrado de angiomiolipoma renal en paciente con síndrome de genes contiguos (TSC2/PKD1) tras 17 años de tratamiento renal sustitutivo. Nefrología. 2017;37:87–92.