Kidney transplant donors lose 50% of their renal mass after nephrectomy. The remaining kidney compensates for this loss and it is estimated that 70% of the baseline renal function prior to donation is recovered. Factors associated with post-donation renal compensation are not well understood.
MethodsRetrospective study of 66 consecutive kidney donors (mean age 48.8 years, 74.2% women). We analyzed the potential factors associated with the compensatory mechanisms of the remaining kidney by comparing donors according to their renal compensation rate (RCR) (Group A, infra-compensation [<70%]; Group B, normal compensation [>70%]).
ResultsWe compared Group A (n=38) and group B (n=28). Predictors for RCR>70% were higher baseline creatinine (A vs. B: 0.73±0.14 vs. 0.82±0.11; p=0.03) and a lower baseline glomerular filtration rate (GFR), estimated both by MDRD-4 (A vs. B: 97.7±18.8 vs. 78.6±9.6ml/min; p<0.001) and CKD-EPI (A vs. B: 101.7±15 vs. 88.3±11.7ml/min; p≤0.001). Age, gender, smoking, hypertension and GFR measured by Tc-DTPA did not show any correlation with the RCR. The multivariate analysis confirmed baseline estimated glomerular filtration rate (eGFR) to be a predictor of compensation: the higher the baseline eGFR, the lower the likelihood of >70% compensation (MDRD-4, OR=0.94 [95% CI 0.8–0.9], p=0.01). The compensation rate decreased by 0.4% (p<0.001) and 0.3% (p=0.006) for every ml/min increase in baseline eGFR estimated by MDRD-4 and CKD-EPI, respectively.
ConclusionsOne year after living donor nephrectomy, the remaining kidney partially compensates baseline renal function. In our experience, baseline eGFR is inversely proportional to the one-year renal compensation rate.
Los donantes renales pierden la mitad de su masa renal tras la nefrectomía. Se estima que el riñón remanente compensa idóneamente un 70% de la función renal previa a la donación. Los factores asociados con el grado de compensación posdonación no están bien establecidos.
MétodosAnálisis retrospectivo de 66 donantes renales consecutivos. Edad media 48,8 años; 74,2% mujeres. Se estudiaron los potenciales factores asociados con la compensación del riñón remanente comparando donantes según su tasa de compensación renal (TCR) (grupo A, infra-compensación [<70%]; grupo B compensación normal [>70%]).
ResultadosComparamos los grupos A (n=38) y B (n=28). Los factores predictores de una TCR>70% fueron una mayor creatinina basal (A vs. B 0,73±0,14 vs. 0,82±0,11; p=0,03) y menor filtrado glomerular (FG), tanto estimado mediante MDRD-4 (A vs. B 97,7±18,8 vs. 78,6±9,6ml/min; p<0,001) como por CKD-EPI (A vs. B 101,7±15 vs. 88,3±11,7ml/min; p≤0,001). La edad, el sexo, el tabaquismo, la hipertensión o el FG medido con Tcm-DTPA no mostraron asociación con la TCR. El análisis multivariante confirmó el FGe como predictor de compensación: a mayor FG basal menor probabilidad de compensar>70% (MDRD-4, odds ratio [OR]=0,94 [IC 95%: 0,8-0,9], p=0,01). La tasa de compensación era 0,4% (p<0,001) y 0,3% (p=0,006), menor por cada ml/min de FG basal más, por MDRD-4 y CKD-EPI respectivamente.
ConclusionesUn año después de la donación renal el riñón remanente compensa parcialmente la función renal basal. En nuestra experiencia el FGe basal se asocia de forma inversamente proporcional a la tasa de compensación renal al año.
Living donor kidney transplantation (LDKT) is the best renal replacement therapy (RRT) for patients with advanced chronic kidney disease (CKD). It provides an improved survival of both graft and patient, and a low rate of surgical complications as it is a programmed intervention.1,2 In Spain, LDKT accounted for 11.4% of all kidney transplants performed in 2016.3 The benefits provided by this type of transplant in terms of morbidity, mortality and quality of life for recipients,4 plus the advances in surgical techniques, preparation and subsequent follow-up of the donor, have made possible to be more flexible in the necessary criteria for renal donation.
Renal donation does not imply biological benefits for those who voluntarily submit to it.
The evolution of renal function, morbidity and mortality in kidney donors has been discussed for years but the literature is not conclusive. Some classic studies with intermediate and long-term follow-up have ruled out a higher risk of CKD or death in renal donors as compared with the general population,5,6 however more recent studies using a better matched healthy control group, find a small increase in the absolute risk of CKD in the very long term, cardiovascular disease and even death in the kidney donor.7,8 This risk could be restricted to donors related to patients with genetic causes of renal disease.7 For all these reasons it is essential to carry out a meticulous and responsible selection process, informing about the risks to each potential donor and preserving the principle of autonomy in each patients.9
One year after donation, the remaining kidney manages to contribute up to 70% of renal function prior to nephrectomy.10 Recent studies indicate that in the remnant kidney, vasodilation and increased renal plasma flow (RPF) occur immediately after nephrectomy. These changes, together with a still not well characterized process of glomerular hypertrophy, make the glomerular filtration of the remaining kidney to increase approximately 40%, without a concomitant increase in the glomerular capillary pressure.11,12 The compensatory capacity of the remaining kidney, and consequently the renal function that the donor reaches are essential to assess the degree of morbidity associated to donation. The objective of our study was to estimate the prognostic value of renal function prior to nephrectomy r to estimate the compensation of subsequent renal function and if there are baseline factors related to the donor that allow us to predict the degree of renal recovery from the remaining kidney. Know the differences in the compensatory response between different subgroups of donors will be useful to inform future donors.
MethodsThis was a retrospective observational study of a cohort of renal donors who consecutively underwent nephrectomy for renal donation between January 2001 and December 2015 at the Hospital del Mar, Barcelona.
The demographic, medical and analytical characteristics were analyzed considering baseline renal function and one year post-nephrectomy of the 66 donors who completed this follow-up period.
Evaluation of renal function pre-donationThe glomerular filtration rate (GFR) was measured by renogram with 99mTc-DTPA between 2 and 6 months before the nephrectomy. The estimated glomerular filtration rate (eGFR) was obtained using the plasma creatinine-based formulas MDRD-4 and CKD-EPI in the live donor study, as described in previous studies of our group.13 The creatinine value closest to the date of the donation was taken as the reference.
Evaluation of renal function one year after donationThe assessment of renal function one year after nephrectomy was made using the MDRD-4 and CKD-EPI formulas. We evaluated the compensation of renal function at one year of donation as the percentage of eGFR reached by the remaining kidney per year with respect to baseline eGF. The calculation was performed using eGFR by MDRD-4, as in other studies,14,15 using the formula:
(Renal compensation rate):(FG at one year of nephrectomy/baseline FG) * 100.
We compared donors with a compensation greater and lower than 70% one year after donation and analyzed baseline characteristics to identify factors that predict the degree of renal compensation.
Evaluation of the absolute change of glomerular filtration rateThe absolute change in eGFR was obtained by subtracting the eGFR one year after donation from the initial eGFR, (calculated by MDRD-4). Given that one year after donation the expected reduction in GFR is between 25 and 40ml/min16 we chose 40ml/min as a cutting point to classify the donors, according to their reduction of FG per year.
Statistic analysisThe quantitative variables with a normal distribution were shown as mean with standard deviation; the categorical variables are presented as frequency/percentage. The variables that did not follow a normal distribution were expressed as median and interquartile range. In all continuous variables the normal distribution was assessed by the Kolmogorov–Smirnoff test. Comparisons were established between the groups described by Student's “t” analysis for continuous variables or Chi-square or Fisher's test for categorical variables. A multivariate analysis was performed using binary logistic regression to evaluate the relationship between different baseline variables and an increase in eGFR or “compensation” of more than 70% one year after nephrectomy, expressed as OR, p-value and 95% confidence interval. A linear regression analysis between continuous basal variables and the rate of compensation was performed. The same analysis was performed for the absolute change of eGFR. To do this, different models were established separating the variables collinearity. All statistical analysis were performed with the SPSS program (SPSS Inc., Chicago, IL) version 20.0. A p<0.05 value was considered statistically significant.
ResultsDonors characteristicsTable 1 shows the baseline demographic, anthropometric and values of renal function of the donor cohort.
Baseline characteristics and renal function one year after nephrectomy in the donor cohort.
| Age at the time of donation (mean±SD, years) | 48.8±10.0 |
|---|---|
| Gender, women (%) | 74.2 |
| Race (n, %) | |
| Caucasian | 58 (87.8) |
| Hispanic | 6 (9) |
| Black | 1 (1.5) |
| Asian | 1 (1.5) |
| Body mass index (mean±SD) | 26.4±3.8 |
| Smoking (n, %) | 27 (41) |
| HTN (n, %) | 4 (6) |
| Obesity (n, %) | 2 (3) |
| Dyslipidemia (n, %) | 22 (33) |
| Basal creatinine (mean±SD; mg/dl) | 0.78±0.14 |
| Baseline MDRD-4 eGFR (mean±SD; ml/min) | 89.32±19.8 |
| eGFR baseline CKD-EPI (mean±SD; ml/min) | 95.4±17 |
| Creatinine after one year mg/dl (mean±SD) | 1.1±0.20 |
| eGFR MDRD-4ml/min per year (mean±SD) | 58.9±10.3 |
| eGFR CKD-EPI ml/min per year (mean±SD) | 65.4±12.6 |
| Absolute change eGFR MDRD-4ml/min per year (mean±SD) | 30.41±16 |
| Absolute change eGFR CKD-EPI ml/min per year (mean±SD) | 30.1±3.5 |
| Rate of Compensation using MDRD (%) | 67.6±13.1 |
| Rate of Compensation using CKD-EPI (%) | 69.2±12.1 |
SD: standard deviation; eGFR: estimated glomerular filtration rate; HTN: arterial hypertension.
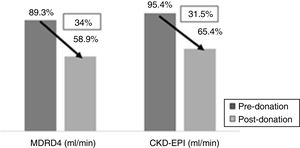
One year after donation, serum creatinine was significantly increased as compared with baseline (1.1 vs. 0.78mg/dl, p<0.001) and a reduction in eGFR as calculated by MDRD-4 (58.9 vs. 89.3ml/min, p<0.001) and by CKD-EPI (65.4 vs. 95.4ml/min, p<0.001). This implies an average loss of 34% of eGFR by MDRD-4 and 31.5% by CKD-EPI in relation to the renal function (Fig. 1).
One year after the donation, by MDRD-4, 2 donors (3%) maintained a eFGR of 30–45ml/min, 38 donors (57.5%) had between 45–60ml/min and 26 (39.4%) had a eGFR>60ml/min. According to CKD-EPI, one donor (1.5%) had eGFR of 30–45ml/min, 26 (39.4%) had eGFR of 45–60ml/min and 39 (59%) with had a GFR>60ml/min.
Evaluation of the rate of compensation to the year of the donation and predictive factorsThe average rate of compensation one year after the donation was 67.6±13.1% as assessed by MDRD4 and 69.2±12.1% by CKD-EPI. The profiles of the donors with compensations higher and lower than 70% after one year of donation were compared (Table 2).
Prognostic factors for a renal function compensation rate greater than 70% after one year (12 m) of donation and evolution of renal function after one year of donation according to compensation rate > or <70%.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| eGFR 12m<70% (N=38) | eGFR 12m>70% (N=28) | p | OR (IC 95%) | p | |
| Donor age (mean±SD, years) | 48.8±11 | 48.9±10.7 | 0.9 | ||
| Gender, women (n, %) | 29 (67) | 19 (86) | 0.08 | ||
| Caucasian race (n, %) | 37 (86) | 20 (87) | 0.4 | ||
| IMC (mean±SD) | 25.6±2.9 | 27.6±4.6 | 0.04 | 1.05 (0.9–1.2) | 0.5 |
| Smoking (n, %) | 20 (47) | 7 (30) | 0.4 | ||
| HTN (n, %) | 1 (2%) | 3 (13%) | 0.08 | ||
| Obesity (n, %) | 3 (7%) | 6 (26%) | 0.09 | ||
| Dyslipidemia (n, %) | 12 (28%) | 10 (43%) | 0.2 | ||
| Basal creatinine, mg/dl (mean±SD) | 0.79±0.14 | 0.83±0.14 | 0.04 | 30 (0.7–1225) | 0.8 |
| basal eGFR by MDRD, ml/min (mean±SD) | 94.5±19.4 | 77.8±15 | 0.01 | 0.94 (0.91–0.99) | 0.01 |
| basal eGFR by CKD-EPI (mean±SD) | 98.6±14.4 | 85.9±15 | 0.01 | 0.95 (0.9–0.99) | 0.01 |
| mGFR: Basal 99mTc-DTPA (mean±SD) | 102.3±24.4 | 96.4±19.7 | 0.4 | ||
| eGFR 12m<70% (N=38) | eGFR 12m>70% (N=28) | p | |
|---|---|---|---|
| Creatinine per mg/dl (mean±SD) | 1.14±0.21 | 1.01±0.16 | 0.02 |
| eGFR MDRD-4ml/min per year (mean±SD) | 57.49±10.6 | 61.7±9.1 | 0.1 |
| eGFR CKD-EPIml/min per year (mean±SD) | 63.6±12.9 | 68.9±11.5 | 0.1 |
| Absolute change eGFR MDRD-4ml/min per year (mean±SD) | 38.5±11.99 | 14.31±9.9 | <0.001 |
| Absolute change eGFR CKD-EPI ml/min per year (mean±SD) | 36.9±8.5 | 16.4±11.1 | <0.001 |
SD: standard deviation; eGFR: estimated glomerular filtration rate; mGFR measured glomerular filtration; HTN: arterial hypertension; BMI: body mass index.
The variables that predict a compensation >70% one year after donation by MDRD-4 were a higher baseline creatinine (A vs. B 0.73±0.14 vs. 0.82±0.11; p=0.03) and lower GFRFG estimated by either MDRD-4 (A vs. B 97.7±18.8 vs. 78.6±9.60ml/min; p<0.001) or CKD-EPI (A vs. B 101.7±15 vs. 88.3±11.7ml/min; p≤0.001). Age, sex, smoking, obesity or BMI, hypertension or dyslipidemia were not significantly associated with the rate of compensation of renal function. Also, the variable FGm-TcDTPA did not show association with the degree of increase in eGFR. Multivariate analysis vas performed using different models that included baseline creatinine and baseline eGFR for MDRD4 or eGFR for CKD-EPI. The baseline eGFR by MDRD-4 remained the only independent predictor of compensation, the inverse relationship being that, the higher the baseline eGFR, the lower the compensatory capacity >70%, (OR=0.94 [95% CI, 0.8–0.9], p=0.04).
The rate of renal function compensation showed a positive correlation with baseline creatinine (r=0.46; p<0.001) and a negative correlation with baseline GFR, both estimated by MDRD-4 (r=−0.73; p<0.001) as per CKD-EPI (r=−0.51; p=0.001). The compensation was 0.4% (p<0.001) and 0.3% (p=0.006) lower for each ml/min of baseline eGFR calculated by MDRD-4 and CKD-EPI respectively.
Table 2 shows the evolution of the eGFR according to the rate of compensation.
Evaluation of absolute change in glomerular filtration rateThe absolute change of eGFR was calculated. The eGFR decreased by 30.41±16 using MDRD-4 and by 30.1±13.5ml/min using CKD-EPI.
Donors were grouped among those with a decrease in eGFR grater or lower than of 40.9ml/min of the baseline GFR. Table 3 shows the differences between the two groups and it is also shown shows renal after one year depending on whether the loss of GFR had been greater or lower than 40ml/min.
Prognostic factors of a loss of eGFR>40ml/min with respect to baseline renal function one year (12m) after donation and the evolution of renal function according to absolute loss of GFR with respect to basal: > or <40ml/min.
| Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|
| Absolute change eGFR 12m>40ml/min | Absolute change eGFR 12m<40ml/min | p | OR (CI 95%) | p | |
| Donor age (mean, years) | 45.6±13.3 | 50±9.6 | 0.1 | ||
| Gender, woman (n, %) | 10 (5.5) | 39 (81.2) | 0.03 | ||
| Caucasian (n, %) | 15 (83.3) | 43 (89.5) | 0.5 | ||
| BMI (mean±SD) | 25.6±2.6 | 26.5±4 | 0.4 | 1.05 (0.9–1.2) | 0.5 |
| Smoking (n, %) | 9 (50) | 18 (37.5) | 0.3 | ||
| HTN (n, %) | 0 (0) | 4 (8.3) | 0.2 | ||
| Obesity (n, %) | 0 (0) | 2 (4.1) | 0.2 | ||
| Dyslipidemia (n, %) | 3 (16.6) | 19 (39.6) | 0.07 | ||
| Basal creatinine, mg/dl (mean±SD) | 0.72±0.12 | 0.80±015 | 0.06 | 30 (0.7–1225) | 0.8 |
| Basal eGFR (MDRD) ml/min (mean±SD) | 101.86±10.7 | 84.62±20.4 | 0.001 | 0.94 (0.91–0.99) | 0.01 |
| Basal eGFR (CKD-EPI) ml/min (mean±SD) | 109.1±12.6 | 90.3±15.6 | <0.001 | 0.95 (0.9–0.99) | 0.01 |
| Basal mGFR, (99m Tc-DTPA) (mean±SD) | 108.7±25.4 | 97.2±20.5 | 0.08 | ||
| Absolute change in eGFR 12m>40ml/min | Absolute change in eGFR 12m<40ml/min | p | |
|---|---|---|---|
| Serum Creatinine after one year, mg/dl (mean±SD) | 1.14±0.21 | 1.01±0.16 | 0.02 |
| eGFR, ml/min (MDRD-4) after one year(mean±SD) | 57.49±10.6 | 61.7±9.1 | 0.1 |
| eGFR, ml/min (CKD-EPI) after one year (mean±SD) | 63.6±12.9 | 68.9±11.5 | 0.1 |
| Absolute change in eGFR (ml/min) after one year (MDRD-4) (mean±SD) | 38.5±11.99 | 14.31±9.9 | <0.001 |
| Absolute change in eGFR (ml/min) after one year (CKD-EPI) (mean±SD) | 36.9±8.5 | 16.4±11.1 | <0.001 |
SD: standard deviation; eGFR: estimated glomerular filtration rate; mGFR measured glomerular filtration rate; HTN: arterial hypertension; BMI: body mass index.
Donors that had lost >40ml/min of baseline eGFR had higher basal GFR by MDRD-4 (101.9±10.7 vs. 84.6±20.4ml/min; p≤0.001) and by CKD-EPI (109.1±12.6 vs. 90.3±15.6ml/min; p≤0.001).
Absolute loss of eGFR showed a negative correlation with baseline creatinine (r=−0.3, p<0.001) and positive correlation with baseline eGFR, MDRD-4 (r=0.5; p<0.001). The absolute loss of eGFR was 0.5ml/min greater for each ml/min of baseline eGFR (p<0.001).
DiscussionIn this study living kidney donors we have analyzed the effect of the renal function prior to nephrectomy as well as other baseline variables on the ability to compensate for subsequent loss of renal function after nephrectomy. The evaluation of the renal compensation rate revealed that donors with higher creatinine, and therefore a lower baseline eGFR, compensated more than those with a higher eGFR. Most published studies analyzed the ability of renal donors to achieve a certain value of eGFR, generally greater than 60ml/min, considering that this function demonstrates an adequate evolution after donation. The proportion of donors that does not reach this value varies according to the series between 10 and 91%.15,16 Studies that assess the baseline factors associated with the recovery of baseline renal function agree that a higher baseline GFR predicts better renal function one year after donation.12 Our results are similar: a higher baseline FG is a predictor of reaching >60ml/min of eGFR a year after donation (data not shown). However, the suitability of this criterion for the evaluation of renal function is not clear. The GFR below 60ml/min which is considered to establish CKD in the general population17 is not applicable to healthy renal donors who, although suffering from a decrease in their renal mass, preserve the function of the remaining kidney.18,19
Numerous studies have described the process by which the remaining kidney increases the GFR after nephrectomy of the contralateral kidney. In humans, immediately after nephrectomy the renal flow increases, so that despite the fact that half of the renal mass is removed, the GFR reaches 70% of the previous renal function.10,12 Recent morphometric studies indicate that the flow increases in parallel to the renocortical volume and the ultrafiltration coefficient, calculated using mathematical models, and conclude that the increase in post-nephrectomy GFR can be explained exclusively by the increase in flow without an increase in glomerular pressure.11
Consistent with previous studies, in our donors, the mean eGFR one year after donation fell to around 30% of the previous GFR.16 Taking into consideration that optimal recovery is to reach 70% of baseline renal function at one year after nephrectomy, our objective was to study the evolution of renal function according to this criterion. Our results show that although donors with a higher baseline GFR are more likely to achieve a eGFR >60C/ml/min after one year, a lower baseline eGFR is associated with greater degree compensation after the completion of one year after donation. The analysis of the absolute loss of eGFR, revealed that the donors who lost >40ml/min of the baseline eGFR started with higher eGFR. This is due to the fact that the donors that compensate the most had a lowest absolute decrease in eGFR. The underlying physiological mechanisms that may explain this finding are unknown. A possibility is that individuals with a lower baseline GFR present more efficient compensation mechanisms (greater vasodilation capacity, greater glomerular hypertrophy, etc.). A limited number of studies in the literature have evaluated the rate of kidney compensation,14,15 therefore more studies are required and with longer follow-up time to analyze whether the renal compensation rate predicts the long-term evolution of renal function.
Apart from baseline renal function, no other characteristics were identified at baseline that could predict an adequate renal compensation rate one year after donation. Although there are studies that assess the importance of some factors to reach a eGFR >60ml/min after nephrectomy,6,16,20 there are only few studies that evaluate prognostic factors of renal compensation rate.15 The results on the influence of the different factors studied are not homogeneous. Some studies have described that patients with a higher body mass index are more likely not to reach a eGFR >60ml/min after nephrectomy,6,12 however this concept is not uniform among the authors.21 Age decreases the number of nephrons and increases the degree of arteriosclerosis.15 Although previous studies indicate that donors older than 60 years have lower renal function before and after nephrectomy,6,22 no significant differences were found in the percent of decrease in renal function as compared to younger donors. Dols et al.23 described that after an initial decrease in the GFR, that was not different in patients older or younger that 60 years, there was no evidence of an accelerated loss of eGFR being no different than in the general population: 5–10ml/min per decade. Recently, another study confirmed that the renal compensation rate does not differ according to age.15 Most studies have not demonstrated an effect of gender per se on the evolution of renal function after nephrectomy.14,20,22 Similarly, several publications associate the African-American race with a worse long-term evolution of renal function,24 although no short-term differences have been demonstrated after nephrectomy. There are also no uniform results about the role of arterial hypertension.20,25
The main limitation of our study is being unicentric retrospective observational and with a limited number of donors. The low ethnic variability and low prevalence of obesity among our donors makes difficult to explore the impact of these factors. In addition the GFR was not measured after one year of nephrectomy because it was not established in our routine clinical protocol.
In our experience, donors with higher creatinine and lower baseline GFR show the highest rate of renal compensation after the same period of follow-up. Only renal function reached significance as a predictor of the compensation rate. Further studies and longer follow-up are necessary to evaluate the suitability of the renal compensation rate as a criterion to evaluate the evolution of renal function in renal donors.
FinancingThis study has been carried out, in part, thanks to the financing of the FIS-FEDER projects PI13/00598, FIS-FEDER PI16/00617 and RETIC Redinren FEDER RD16/0009/0013 (RedinRen).
Conflicts of interestThe authors declare no conflict of interest related to the content of this article
The authors thank Sara Álvarez, Montserrat Folgueiras, Anna Faura, Maria Vera, Raquel Martín, Ernestina Junyent and the entire nephrology nursing team at Hospital del Mar for their ivaluable collaboration in this project.
Please cite this article as: Burballa C, Redondo-Pachón D, Pérez-Sáez MJ, Arias-Cabrales C, Mir M, Francés A, et al. Factores asociados a la compensación de la función renal tras la nefrectomía para donación. Nefrologia. 2018;38:528–534.
The study was carried out as part of Carla Burballa's doctoral thesis at the Universitat Autònoma de Barcelona.