Hyponatremia is one of the most common electrolyte abnormalities in clinical practice. Data regarding factors that have impact on mortality of severe hyponatremia and outcomes of its therapeutic management is insufficient. The present study aimed to examine the factors associated with mortality and the outcomes of treatment in patients with severe hyponatremia.
Materials and methodsPatients with serum Na≤115mequiv./L who were admitted to Ordu State Hospital and Ordu University Training and Research Hospital between 2014 and 2018 were included in the study. Demographic and laboratory features, severity of the symptoms, comorbid diseases, medications, and clinical outcome measures of the patients were obtained retrospectively from their medical records. Factors associated with in-hospital mortality, overcorrection and undercorrection were assessed.
ResultsA total of 145 patients (median age 69 years and 58.6% female) met inclusion criteria. Diuretic use was the most common etiologic factor for severe hyponatremia that present in 50 (34.5%) patients. Sixty-seven (46.2%) patients had moderately severe while 8 patients (5.5%) had severe symptoms. The median increase in serum Na 24h after admission in the study population was 8.9mequiv./L (−6 to 19). Nonoptimal correction was seen in 92 (63.4%) patients. Hypertonic saline use was associated with overcorrection (OR, 3.07; 95% CI: 1.47–6.39; p=0.002). Avoidance of hypertonic saline (aOR, 2.52; 95% CI: 1.12–5.66; p=0.029) and having neuropsychiatric disorder (aOR, 2.60; 95% CI: 1.10–6.11; p=0.025) were associated with undercorrection. In-hospital mortality rate was 12.4% and having CKD and cancer, undercorrection of sodium and presence of severe symptoms were significantly associated with in-hospital mortality.
ConclusionSevere hyponatremia in hospitalized patients is associated with substantial mortality. The incidence of non-optimal correction of serum Na is high; under-correction, presence of severe symptoms, chronic kidney disease and cancer were the factors that increase mortality rate.
La hiponatremia es una de las alteraciones electrolíticas más frecuentes en la práctica clínica. Los datos sobre los factores que tienen impacto en la mortalidad de la hiponatremia grave y los resultados de su manejo terapéutico son insuficientes. El presente estudio tuvo como objetivo examinar los factores asociados con la mortalidad y los resultados del tratamiento en pacientes con hiponatremia grave.
Materiales y métodosSe incluyeron en el estudio pacientes con Na sérico ≤ 115 mequiv./L que ingresaron en el Hospital Estatal de Ordu y en el Hospital de Investigación y Capacitación de la Universidad de Ordu entre 2014 y 2018. Las características demográficas y de laboratorio, la gravedad de los síntomas, las enfermedades comórbidas, los medicamentos y las medidas de resultado clínico de los pacientes se obtuvieron retrospectivamente de sus registros médicos. Se evaluaron los factores asociados con la mortalidad hospitalaria, la hipercorrección y la hipocorrección.
ResultadosUn total de 145 pacientes (mediana de edad de 69 años y 58,6% mujeres) cumplieron los criterios de inclusión. El uso de diuréticos fue el factor etiológico más común para la hiponatremia grave que se presenta en 50 (34,5%) pacientes. Sesenta y siete (46,2%) pacientes tenían síntomas moderadamente graves, mientras que 8 pacientes (5,5%) tenían síntomas graves. El aumento medio del Na sérico 24 h después de la admisión en la población de estudio fue de 8,9 mequiv./L (-6 a 19). Se observó una corrección no óptima en 92 (63,4%) pacientes. El uso de solución salina hipertónica se asoció con sobrecorrección (OR, 3.07; 95% CI:1.47–6.39; p = 0.002). Evitar la solución salina hipertónica (aOR, 2.52; 95% CI: 1.12–5.66; p = 0.029) y tener un trastorno neuropsiquiátrico (aOR, 2.60; 95% CI: 1.10–6.11; p = 0.025) se asociaron con hipocorrección. La tasa de mortalidad hospitalaria fue del 12,4% y tener ERC y cáncer, hipocorrección de sodio y presencia de síntomas graves se asociaron significativamente con la mortalidad hospitalaria.
Conclusiónla hiponatremia severa en pacientes hospitalizados se asocia con una mortalidad sustancial. La incidencia de corrección no óptima del sodio sérico es alta; la corrección insuficiente, la presencia de síntomas graves, la enfermedad renal crónica y el cáncer fueron los factores que incrementaron la tasa de mortalidad.
Hyponatremia (serum Na<135mequiv./L) is one of the most common electrolyte abnormalities in clinical practice and it is associated with increased in-hospital mortality.1–3 Severe hyponatremia per se may lead to life-threatening cerebral edema; it may also reflect the severity of the underlying cause which may impact on mortality. The rate of correction in response to treatment is relatively unpredictable in case of severe hyponatremia. Overly rapid correction of sodium may result in osmotic demyelination syndrome.4,5 There has been a considerable debate on optimal rate of correction of hyponatremia.6,7 Although, two recent consensus guidelines from US and Europe have published recommendations regarding diagnosis and treatment of hyponatremia, there is meager evidence from randomized trials to guide the management of severe hyponatremia.6,8,9 Notably, data regarding factors that have impact on mortality of severe hyponatremia and outcomes of its therapeutic management including non-optimal correction is insufficient. The present study, conducted in relatively large cohort of patients with severe hyponatremia, aimed to examine the factors associated with mortality and the outcomes of treatment.
Material and methodsStudy design and data collectionThis study was designed retrospectively. Ethical approval was obtained from the local ethical committee and the study was conducted in accordance with the ethical standards specified in the Helsinki Declaration. Patients with serum Na≤115mequiv./L who were admitted to Ordu State Hospital and Ordu University Training and Research Hospital between January 01, 2014 and December 31, 2018 and hospitalized for at least 24h were included in the study. Patients who were under the age of 18 were excluded from the study. Along with the demographic and laboratory features, severity of the symptoms at the time of admission, volume status, comorbid diseases, medications, and clinical outcome measures of the patients were obtained from their medical records. The severity of symptoms was classified as “moderately severe” or “severe” in accordance with European practice guidelines on diagnosis and treatment of hyponatremia.9 Treatment modalities used including hypertonic (3%) NaCl were documented. Serum Na levels at the time of admission, follow-up and discharge were recorded. Estimated sodium value at the 24th hour (Na24) was calculated with the formula used by Geoghegan et al.10: Na24=Naa+[(Nab−Naa)×(24−Ta)/Tb−Ta]. In this formula, Naa indicates the closest serum sodium measurement before the 24-h mark; Nab, the closest serum sodium measurement after the 24-h mark, Ta; the time at which measurement Naa was taken, and Tb, the time at which measurement Nab was taken. Overcorrection was defined as the increase in serum Na concentration 24h after admission (Nai24)>10mequiv./L, while undercorrection was defined as Nai24<6mequiv./L.
Statistical analysisData were expressed as medians (minimum–maximum) for continuous variables and percentages for categorical variables. Mann–Whitney U and Kruskal–Wallis tests were used to compare medians for continuous variables. The Chi-square and the Fisher's exact test were used to compare categorical variables. Factors associated with in-hospital mortality, overcorrection and undercorrection were assessed using logistic regression analysis. Data were analyzed by using IBM SPSS version 21. A p value of <0.05 was considered statistically significant.
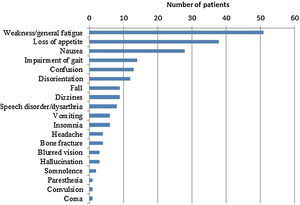
ResultsDemographic and clinical features of study populationA total of 145 patients with a diagnosis of severe hyponatremia (Na<115mequiv./L) over the study period were included in the study. The median age was 69 (21–93) and 80 patients (58.6%) were female. Table 1 lists demographic and clinical features of the study group. Symptoms of hyponatremia were documented in 120 patients (82.8%), while 25 patients had no symptoms. Muscle weakness and general fatigue were the most common symptoms (Fig. 1). Sixty-seven (46.2%) patients had moderately severe while 8 patients (5.5%) had severe symptoms.
Patients’ characteristics and laboratory results at admission.
| Sex (female) (n, %) | 80 (58.6) |
| Median (min.–max.) | |
|---|---|
| Age (years) | 69 (21–93) |
| Sodium (mequiv./L) | 110 (88–115) |
| Potassium (mequiv./L) | 3.9 (2–6.4) |
| Chloride (mequiv./L) | 80 (59.7–121) |
| BUN (mg/dL) | 22.4 (8.6–57) |
| Creatinine (mg/dL) | 1.1 (0.3–3.3) |
| Glucose (mg/dL) | 110 (83–224) |
| Albumin (g/dL) | 3.8 (2–4.3) |
| Uric acid (mg/dL) | 5.5 (1.3–12.1) |
| Urine sodium (mequiv./L) | 67 (14–154) |
| Urine potassium (mequiv./L) | 31 (6–75) |
| ALT (IU/L) | 19 (7–228) |
| Serum osmolality (mosm/kg) | 237 (210–251) |
| Comorbid diseases | n (%) |
|---|---|
| Hypertension | 112 (77.2) |
| Chronic kidney disease | 50 (34.5) |
| Diabetes mellitus | 39 (26.9) |
| Heart failure | 33 (22.8) |
| Neuropsychiatric disease | 35 (24.1) |
| Cancer | 26 (17.9) |
| Cirrhosis | 8 (5.5) |
BUN: blood urea nitrogen; ALT: alanine aminotransferase.
The study population predominantly had chronic hyponatremia; only 8 (5.5%) patients had acute hyponatremia. Hypertension was the most common comorbidity present in 112 patients (77.2%) in the study population; 50 (34.5%) patients had chronic kidney disease and 39 (26.9%) patients had diabetes mellitus (Table 1). Ninety-three (64.1%) patients were using either angiotensin converting enzyme (ACE) inhibitors or angiotensin II receptor (ARB) blockers. Diuretic use was the most common etiologic factor for severe hyponatremia that was present in 50 (34.5%) patients, while 44 (30.3%) patients had syndrome of inappropriate antidiuretic hormone secretion and 35 patients (24.1%) had hypervolemic hyponatremia (Table 2).
Etiology of severe hyponatremia.
| Etiology | n (%) |
|---|---|
| Hypovolemic hyponatremia | 16 (11.0) |
| Hypervolemic hyponatremia | 35 (24.1) |
| Euvolemic hyponatremia | |
| Diuretic-associated hyponatremia | 50 (34.5) |
| Syndrome of inappropriate antidiuretic hormone (SIADH) | 44 (30.3) |
| SSRI | 17 (11.7) |
| Malignancy | 9 (6.2) |
| Anti-epileptics | 5 (3.4) |
| Antipsychotics | 3 (2.1) |
| Other drugs | 3 (2.1) |
| Pneumonia | 1 (0.7) |
| Unknown etiology | 6 (4.1) |
| 145 (100) | |
Thirty-two patients (23.4%) were admitted to intensive care unit (ICU). Hypertonic saline (3% NaCl) was used in 88 (60.7%) patients within 24h of admission. Fifty-one patients out of 75 (68%) with moderately severe or severe symptoms received hypertonic saline. The median increase in serum Na concentration 24h after admission (Nai24) in the study population was 8.9mequiv./L (−6 to 19), it was significantly higher in patients who received hypertonic saline (10.5mequiv./L [−2 to 19] vs. 8.0mequiv./L [−6 to 14], p<0.001). Volume status did not have a significant impact on overcorrection rate (p>0.05). Hypertonic saline use was significantly associated with overcorrection of serum Na (OR, 3.07; 95% confidence interval [95% CI], 1.47–6.39; p=0.002), none of the other demographic or clinical factors were associated with overcorrection. Patients were more prone to undercorrection if they did not receive hypertonic saline (aOR, 2.52; 95% confidence interval [95% CI], 1.12–5.66; p=0.029) or if they had a history of neuropsychiatric disorder (aOR, 2.60; 95% confidence interval [95% CI], 1.10–6.11; p=0.025). Osmotic demyelination syndrome (ODS) was not reported in any patients in the study population including 58 (40%) patients with overcorrection. Seven patients (4.8%) had moderate to severe hypokalemia (serum K <3mequiv./L) at admission and 8 patients (5.5%) had liver cirrhosis. None of the patients had history of alcoholism. In multivariable analysis, having CKD and cancer, Na correction of <6mequiv./at 24h, presence of severe symptoms at admission were significantly associated with in-hospital mortality (Table 3). In-hospital mortality for the entire cohort was 12.4% (18/145). The mortality rate was not significantly different in patients with overcorrection of Na (4/58) compared with the rest (14/87) of the study population (p=0.10). When patients were classified according to their degree of Na correction, patients with undercorrection of Na had the highest mortality rate (26.5%) compared to patients with modest correction (Nai24, 6–10mequiv./L) and the ones with overcorrection. The median length of stay (LOS) at the hospital was 10 (3–43) days. LOS did not differ significantly with the level of Na correction (Table 4). Forty-five patients (35.4%) who survived were still hyponatremic (median Na, 131 [121–134]) at time of discharge while the rest had normal Na levels (median Na, 137 [135–145]).
Factors associated with mortality.
| Variable | Adjusted OR (95% CI) | p |
|---|---|---|
| Na correction of <6mequiv./L at 24th hour | 6.68 (2.00–22.32) | 0.002 |
| Presence of severe symptoms | 4.22 (1.17–15.19) | 0.028 |
| CKD | 8.52 (2.18–33.25) | 0.002 |
| Cancer | 7.02 (1.77–27.78) | 0.005 |
OR, odds ratio; 95% CI, 95% confidence interval; Na, sodium; CKD, chronic kidney disease.
In the present study, we reported the outcome of severe hyponatremia and the factors affecting the outcome in relatively large cohort of hospitalized patients identified over five years. Our findings showed that severe hyponatremia is associated with significant mortality. In addition to clinical presentation of severe hyponatremia per se and its correction rate, we found that associated comorbidities have significant impact on the mortality rate.
Among patients admitted with severe hyponatremia, previous studies reported in-hospital mortality rates that range from 3.9% to 27%.5,11–14 We have found relatively high (12.4%) in-hospital mortality rate for the entire cohort. Although high mortality rate associated with severe hyponatremia suggests a causal relationship,14–16 there is still substantial debate regarding whether the patients die of hyponatremia per se or co-morbid diseases. A population-based cross-sectional study of 14,697 adults from U.S. showed that after adjustment for age, gender and comorbidities, hyponatremia was still associated with significantly increased risk of mortality in all subjects, suggesting a negative effect beyond that of underlying disease.1 Previous studies had revealed that in acute hyponatremia, patients who die more often experience more severe symptoms than who live.5,9 However, it has not been previously shown whether there is a relationship between symptom severity and mortality in patients with chronic hyponatremia. Our findings suggested that besides implications of comorbidities, patients presenting with severe symptoms are more likely die of severe hyponatremia.
A handful of the available literature concluded that 4–6mmol/L increase in serum Na is sufficient to alleviate most serious manifestations of acute hyponatremia.17 European clinical practice guidelines suggested a target of 5mmol/L increase in the first hour in serum sodium concentration for treatment of hyponatremia with severe symptoms, regardless of whether hyponatremia is acute or chronic.9 American expert panel suggests that in chronic hyponatremia, increase in serum Na concentration should be 4–8mmol/L/day for those at low risk of ODS and 4–6mmol/L/day if the risk of ODS is high.8 Both guidelines recommend avoiding an increase in serum sodium concentration of >10mmol/L during the first 24h. In light of limited available evidence and guidelines, similar to the methodology of Geoghegan et al.,10 we defined undercorrection and overcorrection as Nai24<6mequiv./L and Nai24>10mequiv./L, respectively.
We documented that non-optimal correction (overcorrection and undercorrection) of hyponatremia was quite common in patients admitted with severe hyponatremia, accounting for the two-thirds of the patients. Geoghegan et al. who used similar definitions for the correction rates of Na, also reported a high incidence of non-optimal correction of Na (49%) in a cohort of patients with profound hyponatremia.10 However, they did not find any correlation between mortality and non-optimal correction of Na. Giordano et al. observed higher mortality rates in patients with serum sodium correction rate <0.3mmol/h compared to patients with serum sodium correction rate between <0.5mmol/h and ≥0.3mmol/h.18 In a cohort of 1490 patients admitted with serum sodium <120mequiv./L; George et al. reported higher mortality rate within 30 day of hospital admission in patients with Nai24≤8mequiv./L compared to patients with Nai24≥8mequiv./L.3 In our study, we found that undercorrection of sodium was associated with higher mortality rate compared to modest or overcorrection of serum Na. In the study of Geoghegan et al., the presence of comorbid diseases such as congestive heart failure, chronic liver disease and chronic kidney disease was found to be associated with undercorrection of hyponatremia.10 We did not find any correlation between the underlying diseases and correction rate other than neuropsychiatric disease which was associated with undercorrection. Hyponatremia in neuropsychiatric diseases is common and typically is attributable to either medications used or to the disease itself.19,20 However, previous studies have not shown an association of neuropsychiatric disease with the rate of correction of hyponatremia. Our results also revealed that avoidance of hypertonic saline use was also associated with undercorrection.
Geoghegan et al. found that overcorrection did not increase mortality rate and patients were more likely to experience overcorrection if they were younger, had lower admission serum sodium values, experienced seizures, required ICU admission, or received hypertonic saline.10 In the present study, only use of hypertonic saline was related with overcorrection. The incidence of overcorrection was high (40%) in our study population, however similar to the former study, we did not find a relationship between overcorrection and mortality. Although we observed high incidence of overcorrection, none of the patients in the study population suffered ODS. One explanation for this finding could be the low incidence of the comorbidities that had previously shown to carry high risk for development of ODS including hypokalemia, liver cirrhosis and alcoholism.3,6 Although some recent studies3,10 revealed that the incidence of ODS is low even in case of overcorrection of Na, this however does not mean that exceeding the current limits of correction is plausible in the context of previous experience.5,6 Our results showed that one-third of the patients included in the study with moderate or severe symptoms of hyponatremia were not treated with hypertonic saline. Although in the present study, we reported our retrospective real-world experience on outcomes of severe hyponatremia, fear of ODS or development of pulmonary edema in patients with hypervolemic hyponatremia could be the culprits behind avoidance of hypertonic saline use in some of these patients.
Hyponatremia is common and its management is challenging in cancer patients.21 Ectopic secretion of arginine vasopressin, hypovolemia, antineoplastic and palliative drugs, or pain and nausea may be associated with cancer related hyponatremia.22,23 In a large cohort of 1025 patients with solid tumors, Fuca et al. showed that hyponatremia was independently associated with poorer overall survival (median survival of 2 months versus 13.2 months for patients with and without hyponatremia, respectively).24 Abu Zeinah et al. showed that cancer patients with moderate-severe hyponatremia (Na<130mmol/L) were 4.28 times more likely to die than those with normal-mild hyponatremia (Na≥130mmol/L).25 We documented an independent association between severe hyponatremia and mortality in the setting of cancer in accordance with the available literature.26,27
Patients with CKD may be more susceptible to the development of Na disorders due to their decreased ability to maintain water homeostasis.28 It is not clear whether hyponatremia affects mortality in CKD patients. Lim et al. found that hyponatremia (Na<135mequiv./L) was not associated with all-cause mortality in CKD patients treated with diuretics29 while Kovesdy et al. found that hyponatremia (Na<130mequiv./L) was associated with mortality independent of comorbid conditions such as congestive heart failure or liver disease.30 A recent meta-analysis showed that hyponatremia was associated with increased all-cause mortality in CKD patients.31 In a cohort of ambulatory 45,333 patients with Stage 3 and 4 CKD, Huang et al. concluded that hyponatremia was associated with higher risk for all-cause of death, after adjusting for various confounding variables.32 Our results indicate that in patients with severe hyponatremia, having CKD significantly increases in-hospital mortality independent of all other factors.
The present study was conducted before coronavirus disease-2019 (COVID-19) pandemic. However, the clinical impact of hyponatremia on prognosis of COVID-19 infection has gained interest with time. There have been case reports of euvolemic and hypovolemic hyponatremia secondary to COVID-19.33,34 Bernie at al. showed that hyponatremia was inversely related to blood IL-6 levels and directly related to PaO2/FiO2 ratio in patients with COVID-19.35 Recently, in a cohort of 408 patients with COVID-19, Tezcan et al. found high incidence of hyponatremia (35.8%) and concluded that hyponatremia was an independent factor related to death.36 These preliminary results showed that presence of hyponatremia might reflect a more advanced disease in COVID-19 and impact treatment decisions.
Although we included relatively large number of patients in the present study and limited our analysis to patients with data on outcome of only severe hyponatremia in hospitalized patients, several limitations of our study deserve mention. First, the study design was retrospective. Second, cranial MRI was performed only if there was a clinical suspicion of ODS. There were no autopsy studies, so some of the clinically subtle cases of ODS may have been missed leading to underestimation of true incidence of it. Third, our patients had predominantly chronic hyponatremia, our findings may not be applicable to patients with acute hyponatremia, thus should be interpreted in the appropriate context.
ConclusionsWe have shown that severe hyponatremia in hospitalized patients is associated with substantial mortality. The incidence of non-optimal correction of hyponatremia is quite high; under-correction, presence of severe symptoms, chronic kidney disease and cancer were the factors that appear to increase mortality rate. Our results stress that undercorrection due to concerns for overcorrection in patients with severe hyponatremia may inadvertently increase mortality.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone.
None.