To the Editor,
Extracapillary glomerulonephritis (EGN) is characterised by the presence of potentially pathogenic antibodies or immune complexes in the plasma. These include the antiglomerular basement membrane antibodies (anti-GBM Ab), characteristic of type I EGN, and anti-neutrophil cytoplasmic antibodies (ANCAs), which are usually present in type III EGN.1,2
A substantial proportion of patients with EGN are double positive for anti-GBM and ANCA. We report a patient with type I EGN with plasma coexistence of anti-GBM Ab and p-ANCA and severe renal impairment. The patient did not respond to triple therapy with plasmapheresis, corticosteroids and cyclophosphamide.
Case report
A 62-year old man was referred from the emergency department to the nephrology department for general discomfort associated with bilious vomiting and evening fever. He was taking azithromycin. The patient reported a reduction in diuresis in the previous days, without lower urinary tract symptoms. He was not taking NSAIDs or any other nephrotoxic drugs. The medical history showed chronic obstructive pulmonary disease, obstructive sleep apnoea syndrome, chronic otitis media monitored by an otolaryngologist and botulism, which required ICU admission in 2007. No history of hypertension, diabetes mellitus, dyslipidaemia or any known heart disease. The physical examination revealed a normal general state, he was eupneic at rest, conscious, oriented, collaborative, with normal perfusion and hydration. BP was 143/95mm Hg, HR was 75 beats/min, temperature 36.5°C, and oxygen saturation 97%. There were no lesions on the skin or in the mucous membranes.
Cardiopulmonary auscultation: rhythmic without audible murmurs. Vesicular murmur without superimposed noise was found. Abdomen: soft and palpable. No signs of peritoneal irritation. No masses or organomegaly on palpation. The lower extremities showed pitting oedema to the root of the limbs, without signs of deep vein thrombosis (DVT) or chronic venous insufficiency, with positive pulses. The neurological examination found isochoric and normally reactive pupils, normal cranial nerves, and segmentally conserved strength and sensitivity. There were no signs of meningeal irritation.
The analytical results on admission showed: haemoglobin 11g/dl, haematocrit 34.7%, MCV 92fl, WBC 9000 (85% neutrophils, 9.1% lymphocytes), platelets 213 000, glucose 102mg/dl, urea 265mg/dl, creatinine 12.2mg/dl, sodium 137mEq/l, potassium 7mEq/l, chloride 107mEq/l, procalcitonin 0.92mg/dl, protein 7g/l, calcium 9.6mg/dl, corrected calcium 9.8mg/dl, CRP 272.8, total bilirubin 0.6mg/dl, AST 19U/l, ALT 39U/l, GGT 28U/l, ALP 61U/L, amylase 84. Urinalysis: protein 100mg/dl, 300 red cells, negative nitrites, no leukocyte. Chest x-ray showed no signs of condensation or leakage, and normal mediastinum. The plain abdominal x-ray showed non-specific meteorism.
Venous blood gases: metabolic acidosis with pH 7.28, total CO2 20mmol/l, HCO3-18.8mmol/l, BE -7.5. ECG: sinus rhythm at 75 beats/min, normal PR, narrow QRS, no acute repolarisation changes. Abdominal ultrasound: liver and spleen without ultrasound abnormalities. Enlarged right kidney (15cm), with loss of cortico-medullary differentiation, no observed dilation of the excretory tract. Hypoplastic left kidney (described in previous studies). 99mTc-MAG3 renal scan: non-functioning left kidney. Severe parenchymal nephropathy in the right kidney. Immunology: IgG 795mg/dl, IgA 123mg/dl, IgM 25.1mg/dl, C3 133mg/dl, normal C4, kappa chains 161mg/dl, normal lambda.
Autoimmunity: ANA, anti-DNA Ab and c-ANCA negative, p-ANCA 41U/ml and anti-GBM Ab 287U/ml. Normal serum protein. Serology for HBV and HCV negative.
Given these clinical and analytical findings, a renal biopsy was performed via open lumbotomy, as the patient had a single functional kidney.
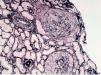
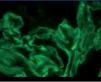
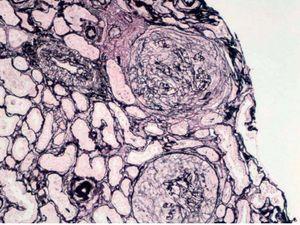
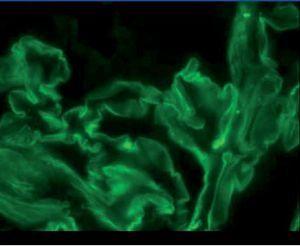
The anatomical pathology described a total of 49 glomeruli, three of them sclerosed and the structure of the others was extensively affected. Fibrinoid necrosis and extracapillary cell and circumferential proliferation were found in 100% of the glomeruli. The small vessels also had necrotising vasculitis. The direct IF study provided a linear and diffuse positive in the glomerular basement membrane, with IgG positive and the rest of the antibodies negative. In summary, extracapillary glomerulonephritis was detected with intense involvement of 100% of the glomeruli of the sample and IF typical of anti-GBM Ab-mediated extracapillary glomerulonephritis (type I), see Figures 1 and 2.
From admission, the patient required renal replacement therapy. He was treated with intravenous cyclophosphamide at a dose of 1.5mg/kg/day, methylprednisolone 500mg/24h for 3 days and plasmapheresis (7 sessions). The cyclophosphamide was discontinued due to thrombocytopaenia. There was no pulmonary involvement at any time during evolution.
Currently, the patient is on haemodialysis with arteriovenous fistula as the vascular access.
Discussion
The percentage of serum coexistence of p-ANCA and anti-GBM Ab was quantified in different series at around 25%.1,3-5 Currently, the clinical profile, the prognosis and the pathophysiological role of each antibody in the serum of patients with p-ANCA and anti-GBM Ab coexistence is still under investigation. It is not clear whether the ANCA-associated GN predisposes the development of the anti-GBM Ab-mediated disease or if the ANCA becomes positive in the course of the anti-GBM Ab-mediated GN.
However, one of the most relevant factors is that IF studies are not routinely described in the literature. Therefore, we are not sure if “double positive” cases are type III or type I EGN.
In type III ANCA+ EGN, the existence of anti-GBM Ab has been described in up to 5% of cases. Due to the greater frequency of this disease, most reported cases are included in this group. Some studies suggest that ANCA GN appears first, followed by anti-GBM Ab disease.1,3 In these cases, it has been suggested that there is an exposure of the glomerular basement membrane antigens and the development of anti-GBM Ab.
In type I EGN, it has been reported that up to 30% of cases may be associated with the existence of ANCA. Due to the lower frequency of this disease, fewer cases have been reported.4,5 The pathogenesis of this condition is not clear and it seems that altered immune regulation results in the development of both, p-ANCA and anti-GBM Ab.
In addition, it has been suggested that patients with ANCA and anti-GBM Ab, paradoxically, have a better prognosis.6 However, this has not been confirmed, and it has even been stated that renal survival in patients with dual positive is no better than those with only anti-GBM Ab.1,3,8 In a study by Lindic et al.,10 patients with anti-GBM Ab, ANCA and a creatinine of higher than 5.6mg/dl on admission did not recover renal function, despite treatment with prednisolone, cyclophosphamide and plasma exchange.
We reported a case of type I EGN with positive linear IF and serum coexistence of anti-GBM Ab and p-ANCA. The poor prognosis of renal function in these cases was seen in our patient, who was dependent on haemodialysis despite aggressive treatment.
Figure 1. Silver technique
Figure 2. Immunofluorescence