Multiple myeloma is a clonal proliferation of plasma cells that causes kidney involvement in 40% of cases, although only 12–15% of cases start with acute kidney failure, with cast nephropathy being the most common cause of this. When acute kidney failure occurs, it requires chemotherapy treatment as well as apheresis techniques in a high percentage of cases1,2 aimed at reducing the concentration of free light chains (FLC) in plasma as early as possible, and thereby preventing their deposition in the kidney tubules.3,4
The emergence of high cut-off haemodialysis (HCO-HD) was postulated as a breakthrough compared to conventional high-flux haemodialysis (HF-HD) due to a faster withdrawal of circulating FLCs. However, preliminary results from two recent studies generate a certain level of controversy.5,6
Recently, medium cut-off (MCO) membranes have been developed, with an ability to eliminate molecules like the HCOs, but able to retain albumin,7–9 which may be a more cost-effective alternative for the adjuvant treatment of acute kidney failure due to kappa myeloma.
We describe the cases of three patients who developed acute kidney failure secondary to kappa FLC tubular deposition (22.5kDa). In all of them, chemotherapy treatment was started early with bortezomib and dexamethasone, according to the haematology protocol, as well as 6-h haemodialysis sessions with the MCO dialyser Theranova 500® by Baxter.
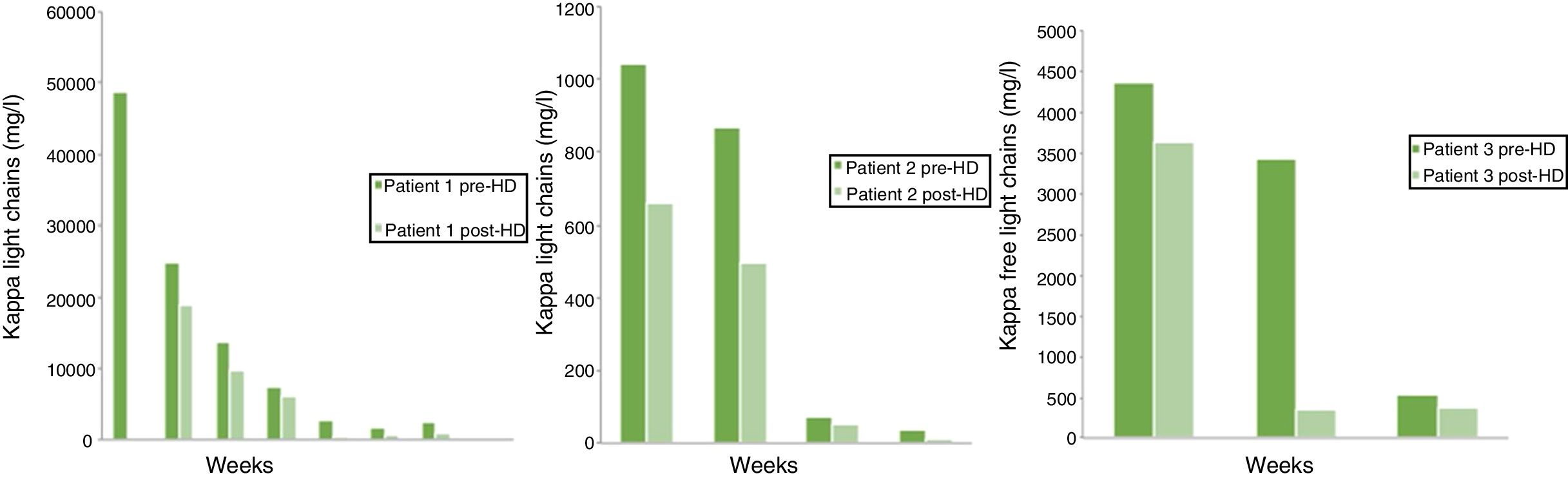
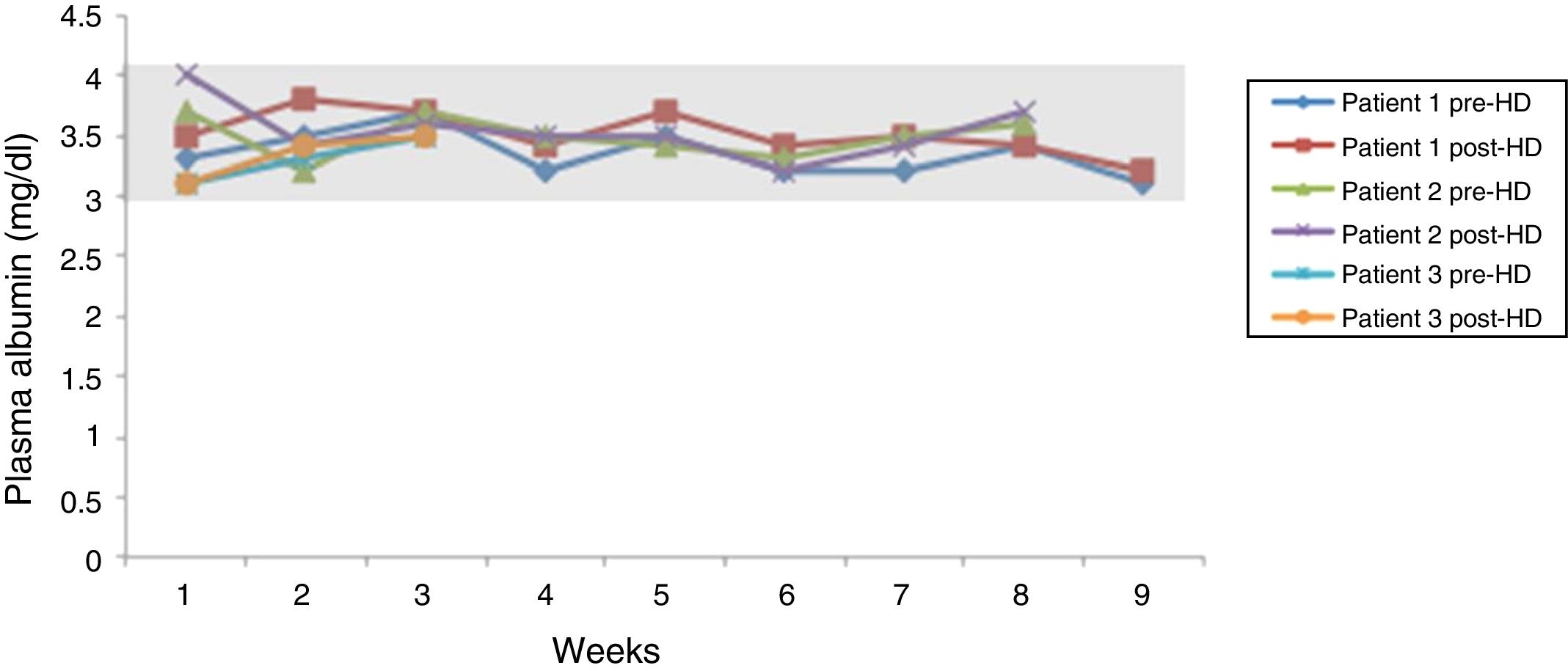
The weekly determination of pre-dialysis creatinine, of FLC kappa (Fig. 1) and of albumin before and after each haemodialysis session (Fig. 2) was carried out.
Case 1A 46-year-old woman in whom hypercalcaemia was detected (15.6mg/dl), kappa FLC levels of 48,900mg/l and deterioration of kidney function (creatinine of 10.3mg/dl). The diagnosis of multiple myeloma was confirmed by bone marrow biopsy (76% of plasma cells).
After 27 sessions of haemodialysis, kidney function remained stable without the need for dialysis and undetectable FLC levels.
Case 2A 72-year-old male with acute kidney failure (creatinine 4.7mg/dl) and elevated kappa FLC levels (1040mg/l). The diagnosis of multiple myeloma was confirmed in a bone marrow biopsy with 14.37% of plasma cells.
The dialysis sessions were spaced out over time according to the analysis. After 32 sessions, the plasma creatinine levels stabilised around 3.4mg/dl, without the need for dialysis.
Case 3A 73-year-old male who presented with acute kidney failure (creatinine 4.6mg/dl), hypercalcaemia (Ca 11.5mg/dl), hyperproteinaemia (14.11g/dl) and kappa FLC levels of 14,300mg/l. The diagnosis of multiple myeloma was confirmed by bone marrow biopsy with 28.66% of plasma cells.
He received seven haemodialysis sessions after which the free light chain levels were reduced to 340mg/l, and kidney function recovered to maintain creatinine levels of 1.4mg/dl, without new dialysis sessions.
In the three cases described, a sustained decrease in kappa chain concentration pre- and post-dialysis was observed, with a mean reduction of 44.8±19.5% in each session, without a significant reduction in the plasma albumin levels. All the patients recovered kidney function without the need for more dialysis sessions.
The development of new chemotherapy treatments and of apheresis, and their early administration has led to an improvement in survival and has increased the renal recovery rate in patients affected by acute kidney failure secondary to cast nephropathy. With the development of haemodialysis with new dialysers, firstly HCO-HD and subsequently MCO-HD, there has been an attempt to improve these results.
Preliminary results of two multicentre, randomised and controlled studies which compare chemotherapy treatment along with HF-HD or HCO-HD deliver contradictory results; while better renal outcomes for patients are shown in the MYRE study,6 the EULITE study5 does not provide statistically significant results. The study of the complete results will be necessary to understand the reason for these differences.
From our experience, the introduction of haemodialysis with medium cut-off dialysers has managed to reduce the loss of albumin which was produced with previous high cut-off dialysers, without this influencing the rapid and effective reduction of the plasma concentration of kappa light chains, obtaining good clinical outcomes and renal survival in treated patients.
Please cite this article as: Cazorla López JM, García García-Doncel A, Naranjo Muñoz J, Villanego Fernández F, Vigara Sánchez LA, Ceballos Guerrero M. Experiencia de hemodiálisis con dializador de mediano poro en la nefropatía por cilindros del mieloma. Nefrologia. 2020;40:367–369.