Objetivo: Comparar la prevalencia y clasificación de la enfermedad renal crónica (ERC) según la estimación del filtrado glomerular (eFG) mediante MDRD-4 IDMS y CKD-EPI en individuos ≥ 60 años en Atención Primaria. Material y métodos: Estudio observacional descriptivo transversal. Sujetos ≥ 60 años atendidos en 40 centros de Atención Primaria con determinación de creatinina sérica realizada entre el 1 de enero y el 31 diciembre de 2010 en un único laboratorio centralizado. Criterios de exclusión: trasplante renal, atención domiciliaria. Variables: sociodemográficas, antropométricas, factores de riesgo y enfermedad cardiovascular según registro en historia clínica electrónica y concentración de creatinina sérica según el método Jaffé cinético compensado estandarizado con IDMS y eFG mediante MDRD-4 IDMS y CKD-EPI. Se analizó la concordancia mediante índice kappa y método gráfico Bland-Altman. Resultados: 97 554 individuos (57,3 % mujeres, mediana de edad 70,0 [Q1: 65,0; Q3: 77,0]). Mediana eFG con MDRD 78,7 [66,7; 91,0] ml/min/1,73 m2 (77,9 en mujeres, 79,7 en varones; p < 0,001) y 81,8 [68,5; 90,5] ml/min/1,73 m2 (p = 0,311) con CKD-EPI; prevalencia eFGMDRD < 60, 15,0 % (16,5 % en mujeres, 13,1 % en varones; 6,5 % en ≤ 70 años, 24 % > 70 años), con CKD-EPI 14,2 % (15,0 % mujeres, 13,0 % varones; 4,7 % en ≤ 70 años, 24,1 % en > 70 años). Se observó una concordancia global del 85,6 % (índice kappa = 0,75), en mujeres > 70 años del 86,6 % (kappa = 0,77), del 83,2 % (kappa = 0,69) en varones > 70 años, del 82,7 % (kappa = 0,68) en mujeres ≤ 70 años y del 90 % (kappa = 0,81) en varones ≤ 70 años. Conclusiones: CKD-EPI disminuyó la prevalencia de ERC especialmente en mujeres ≤ 70 años; la prevalencia aumentó en varones > 70 años. Uno de cada ocho individuos en estadio 3a fue reclasificado a no enfermedad; los individuos reclasificados presentaron menor comorbilidad.
Objective: To compare the prevalence and classification of chronic kidney disease (CKD) in accordance with the estimated glomerular filtration rate (eGFR) by MDRD-4 IDMS and CKD-EPI in individuals ≥ 60 years of age in primary care. Material and Methods: Cross-sectional descriptive observational study. Subjects ≥ 60 years treated at 40 primary care centres with serum creatinine determination conducted between 1 January and 31 December 2010 at a single centralised laboratory. Exclusion criteria: renal transplantation, home care. Variables: socio-demographic, anthropometric, risk factors and cardiovascular disease as recorded in electronic medical records and serum creatinine concentration by a standardised compensated kinetic Jaffe method with IDMS and eGFR by MDRD-4 IDMS and CKD-EPI. Agreement was analysed using the kappa coefficient and the Bland-Altman graphical method. Results: 97 554 individuals (57.3% women, mean age 70.0 [Q1: 65.0, Q3: 77.0]). Median eGFR with MDRD 78.7 [66.7, 91.0] ml/min/1.73m2 (77.9 for women, 79.7 for men, P<.001) and 81.8 [68.5, 90.5] ml/min/1.73 m2 (P=.311) with CKD-EPI, eFGMDRD prevalence <60 15.0% (16.5% women, 13.1% men and 6.5% in ≤ 70 years, 24%> 70 years) with CKD-EPI 14.2% (15.0% female, 13.0% male, 4.7% in ≤ 70 years, 24.1% in> 70 years) . There was an overall agreement of 85.6% (kappa coefficient = 0.75) in women> 70 years of 86.6% (kappa = 0.77), of 83.2% (kappa = 0.69) in men> 70 years, of 82.7% (kappa = 0.68) in women ≤ 70 years and 90% (kappa = 0.81) in men ≤ 70 years. Conclusions: CKD-EPI decreased the prevalence of CKD especially in women ≤ 70 years; the prevalence increased in men> 70 years. One in eight individuals with stage 3a was reclassified to no disease; reclassified individuals had lower comorbidity.
INTRODUCTION
Since the Kidney Disease Outcome Quality Initiative (KDOQI) proposed the new diagnostic criteria for chronic kidney disease (CKD) in 2002,1 CKD has been the subject of multiple studies and has been recognised as a cardiovascular risk factor.2-4
The equation initially recommended for estimating the glomerular filtration rate (eGFR) was that from the MDRD (Modification of Diet in Renal Disease) study known as MDRD-4, which uses four variables (creatinine, age, sex and race) for the calculation.5 Later, after standardisation of creatinine measurements, the recommended equation was the MDRD-4 IDMS equation with the same variables with the previous equation but in this instance it included a correction factor when the creatinine measurement method was shown to be traceable when compared to the isotopic dilution mass spectrometry reference method.6 The most important limitations when using this method are systematic imprecision and underestimation when the eGFR values are higher than 60ml/min/1.73m.2 7
Later, a modification of the MDRD equation, the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI),8 showed a reduction in the bias from the MDRD-4 IDMS equation with improvement in overall imprecision, especially significant in the range of values between 60-89ml/min/1.73 m2; the CKD-EPI equation may replace the MDRD-4 IDMS equation in routine clinical practice in the future.9-12
The objective of this article is to compare the prevalence and classification by CKD stages, according to GFR, using the MDRD-4 IDMS and CKD-EPI equations in individuals aged 60 years or older in Primary Care (PC) and to study the differences according to age and sex, as well as an analysis of the concordance between the two equations.
This study is performed within the framework of a three-year prospective cohort study with the objective of quantifying the risk associated with the decrease in eGFR, calculated by MDRD-4 IDMS and CKD-EPI, in the incidence of cardiovascular events in our area.
MATERIAL AND METHOD
Design
Observational cross-sectional descriptive study at baseline of a prospective three-year follow-up cohort study.
Subjects
Inclusion criteria: Individuals ≥60 years seen in 40 PC centres in the Barcelona metropolitan area who are covered within the Costa de Ponent Primary Care Area (Southern Metropolitan District of the Health Institute of Catalonia) with serum creatinine measurements between January 1 and December 31, 2010.
Exclusion criteria: individuals with renal transplant or those included in the home health program according to their electronic medical record (EMR).
Variables
Sociodemographic and anthropometric variables, coded cardiovascular disease diagnoses (ischaemic heart disease, cerebral vascular accident, peripheral arterial disease, heart failure and atrial fibrillation) and cardiovascular risk factors (hypertension [HTN], diabetes mellitus [DM] and dyslipidaemia) were extracted from the EMR.
Evaluation of renal function
The serum creatinine level was measured by a single laboratory using the standardized Jaffe compensated kinetic method regarding the IDMS reference method.13 The eGFR was carried out using two equations, MDRD-4 IDMS and CKD-EPI, without correction for race, not available. In individuals with more than one serum creatinine measurement, the last measurement was chosen.
MDRD-4 IDMS equation:
eGFR =175 x (creatinine/88.4)-1,154 x (age)-0,203 x (0.742 if female) x (1.210 if black race)
CKD-EPI equation:
For females:
If creatinine ≤ 62µmol/l, eGFR = 144 x ([creatinine/88.4/0.7]-0,329) x 0.993age
If creatinine >62µmol/l, eGFR = 144 x ([creatinine/88.4/0.7]-1,209) x 0.993age
For males:
If creatinine ≤80µmol/l, eGFR = 141 x ([creatinine/88.4/0.9]-0.411) x 0.993age
If creatinine >80µmol/l, eGFR = 141 x ([creatinine/88.4/0.9]-1.209) x 0.993age
The eGFR stages were classified based on the revised criteria of the Kidney Disease Improving Global Outcomes (KDIGO) foundation.14
Statistical analysis
The continuous variables are described with the median and interquartile range (Quartile 1 - Quartile 3 [Q1-Q3]). Normality of the continuous variables was evaluated using normal distribution graphs (histogram, box-plot and Qplot). Qualitative variables were expressed by frequency and percentage. For comparison between sexes, the Mann-Whitney U test was used for comparing quantitative variables and the χ2 test was used for categorical variables. Concordance was evaluated by subgroups for age and sex, according to stages, between the two eGFR methods using the kappa coefficient and the respective 95% confidence interval. Concordance was analysed quantitatively between methods using the intraclass correlation coefficient. Once the individuals who had values greater than 90ml/min/1.73m2 by MDRD-4 IDMS were excluded, the Bland-Altman graph was created to represent the differences between the two methods quantitatively. The concordance limits for the graph were fixed at 95%. The statistical package used was R version 2.14.2 (R Foundation for Statistical Computing Vienna, Austria).
The study was approved by the Jordi Gol Primary Care Research Institute Ethics Committee (IDIAP Jordi Gol).
RESULTS
Of the 189 148 individuals ≥60 years of age who were assigned to the PC centres, 175 867 were seen. This number decreased to 170 039 after excluding patients with kidney transplants and those in the home health program. A serum creatinine level performed between 1 January and 31 December 2010 was available in 97 554 individuals and these were included in the study.
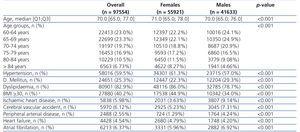
The characteristics of all individuals included in the study are shown in Table 1 by sex. The median of age in our population was 70 years (Q1: 65.0; Q3: 77.0). Women, who made up 57.3% of all individuals, were older and had a higher prevalence of HTN, dyslipidaemia, obesity and heart failure (P<.001); men had a higher prevalence of DM, ischemic heart disease, cerebrovascular accident, peripheral arterial disease and atrial fibrillation (P<.001).
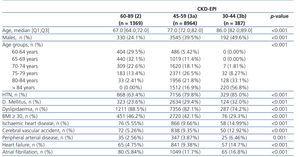
Table 2 shows the creatinine and eGFR results according to the two equations, MDRD-4 IDMS and CKD-EPI, of all individuals grouped by sex and by age subgroups (≤70 years and >70 years). The median creatinine level was significantly lower in women when compared to men: 65 and 83µmol/l, respectively (P<.001). The global median eGFR was higher when the CKD-EPI equation was used than it was with the MDRD-4 IDMS: 81.8 y 78.7ml/min/1,73m2, respectively. The same tendency was seen overall by sex and in individuals that were 70 years of age or lower, but not in men over 70 years of age. When comparing women to men, when the MDRD-4 IDMS equation was used, the median eGFR was lower in women, 77.9 and 79.7, respectively (P<.001) with no significant differences with the CKD-EPI equation (0.311).
Conversely, when comparing the median eGFR by CKD-EPI by sex, stratified by age subgroups (≤70 years and >70 years), there were significant differences with higher values for younger women (P<.001) and older men (P<.001).
The overall prevalence of eGFR <60 decreased from 15% when the MDRD-4 IDMS equation was applied to 14.2% with CKD-EPI. The decreased was primarily due to women, where it went from 16.5% to 15%, with hardly any variation in men (from 13.1% to 13%, respectively). In individuals older than 70 years of age, the overall prevalence of eGFR <60 was similar with both equations. However, a contrary tendency was detected by sex: it decreased in women from 26.2% to 25.4% and increased in men from 20.7% to 22.3%. A decreased was seen in the distribution by stages with CKD-EPI in stage 3a from 11% to 9.8%, especially in women, and a slight increase was seen in more severe stages.
When comparing the classification of individuals by eGFR stage, taking the MDRD-4 IDMS as a reference, the overall percentage of concordance was 85.6% with a kappa coefficient of 0.75 (0.74; 0.75) (Table 3). The percentage that coincided was greater in stages 4 and 5 and less in stages 1 and 3a. When stratifying by sex and age, greater concordance was observed in the subgroup of men 70 years of age or less with a kappa coefficient of 0.81 (0.81; 0.82) and least in the subgroup of women with the same age interval, which had a kappa coefficient of 068 (0.68; 0.69). Concordance was lower in all subgroups in stage 3a than in 3b, 4 and 5; the low coincidence in stage 1 was only observed in men and women older than 70 years.
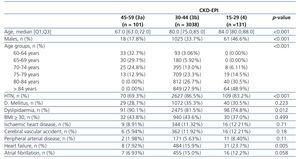
In stage 3a, the greatest discrepancy was found in the 70 years and under age subgroup, where 41.3% of women and 24.4% of men were reclassified to stage 2.
When studying the characteristics of the individuals reclassified to no disease (stage 3a by MDRD-4 IDMS and stage 2 by CKD-EPI), a greater percentage of younger women with a lower incidence of risk factors and cardiovascular disease were observed (Table 4) with statistically significant differences. The same was observed in the subgroups of individuals that went from stage 3b by MDRD-4 IDMS to stage 3a by CKD-EPI, but statistically significant differences were only seen for age, sex, HTN and heart failure (Table 5).
Figure 1 shows the concordance between methods using a dispersion graph and the Bland-Altman graph stratified by sex for individuals 70 years of age and under and MDRD-4 IDMS eGFR values less than 90ml/min/1.73m2. The dispersion graph shows that both men and women had greater overall values on CKD-EPI. The observed values were clearly higher than those expected for equality of the values between equations. Women had slightly greater values compared to men. The Bland–Altman graph revealed that the mean overall differences between equations was 4.5 units greater in the CKD-EPI estimation with 95% agreement limits that fluctuated from 8.3 to 0.63ml/min/1.73m2 (greater in CKD-EPI). As the eGFR increased, the differences between equations increased in favour of the CKD-EPI method. Figure 2 shows the same analysis for individuals over 70 years of age, also stratified by sex. The dispersion graph between equations revealed how the two types of estimations had similar values for both men and women given that the observed and expected values were very similar. Women had values that were slightly higher than those of men with regards to the CKD-EPI equation. For the Bland-Altman graph, the mean of the differences between equations for all individuals was 0; indicating no average systematic variability. The concordance limits were observed between 4.3 and -4.3ml/min/1.73m2. This graph also shows how the variability between equations grew with the increase in eGFR.
DISCUSSION
In our population of individuals 60 years of age or older or those who were seen more in PC, the eGFR calculation by CKD-EPI decreased the overall percentage of eGFR <60 by 5.3% in relative terms (from 15 to 14.2%). The greatest decrease occurred in women 70 years of age or younger (reduction of 35%) and in least occurred in women >70 years of age (3%); the prevalence increased by 7.7% in men >70 years. The decrease in prevalence obtained with CKD-EPI was concentrated in stage 3a, with a slight increase in the remaining stages. 1.4% of individuals were reclassified to no disease (eGFR > 60), all of whom were from stage 3a. This represents 12.8% of individuals in this stage. The reclassification had a particular impact on individuals younger than 70 years of age (33.6% of individuals in stage 3a versus 5.6% in those over 70 years of age) and more in women (41.3%) than in men (24.4%). The reclassified individuals had a lower prevalence of risk factors and cardiovascular disease.
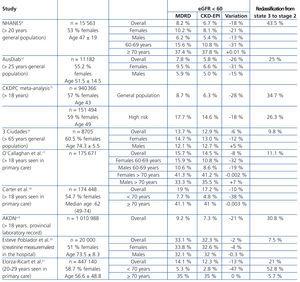
Table 6 shows the estimated prevalences for eGFR <60 by MDRD and CKD-EPI in different studies. These reveal a reduction in overall prevalence with CKD-EPI which varies from 2% to 28% depending on the characteristics of the population.8,10,15-21 The decrease is greater in study populations with lower prevalences of eGFR <60, usually in younger subjects, which could be related to the lower baseline risk level and concordant with that seen in two populations, general and high risk, on the CKDPC meta-analysis.15
Women had a greater reduction than men and, in both sexes, the difference between the two formulas decreased with age, even observing an increase in the prevalence of eGFR <60 with CKD-EPI in men starting at 65 or 70 years of age, depending on the study.
The decrease in the prevalence of eGFR <60 observed with CKD-EPI, as with other studies, was concentrated in the milder stage, stage 3. The percentages of subjects that were reclassified with CKD-EPI from stage 3 to eGFR ≥60 varied in the different studies from 7.5% to 43.5%. In general, studies with a greater reduction in the prevalence with CKD-EPI also had more reclassification. In study 3 by Ciudades,16 with subjects over 65 years of age with risk factors similar to our population, the reduction in the total prevalence of eGFR <60 and reclassification to eGFR ≥ 60 were similar to those seen in our study.
In studies which analyse the characteristics of reclassified individuals,8,10,17-19 these were younger and with a higher proportion of women. The AKDN also presented lower comorbidity such as HTN or DM.
In the study by O'Callaghan17 in subjects under 75 years of age, including men and women, there was a net change to a higher eGFR, i.e. a lower stage. Conversely, in those over 80 years of age, the change was to a lower eGFR and worse stage. Carter et al.18 described a lower difference in eGFR estimation between MDRD-4 IDMS and CKD-EPI at ages between 70-79 years and among the much older subjects, the CKD-EPI obtained higher estimations that MDRD-4 IDMS.
The decrease in prevalence seen in our study, though somewhat lower, is concordant with studies that used similar methodology, the same age groups and standardisation of creatinine measurement, as well as the percentage that was reclassified to stage 3.
In the studies carried out in our country, Montañes et al.22 compared the use of both formulas in individuals between 18-97 years of age seen in a nephrology-urology clinic and they also found greater concordance between both formulas in men and in those over 70 years of age. The percentage of reclassification from stage 3a to no disease was 17% overall and 34.1% in those under 70 years of age. In addition, 9.8% of cases catalogued as stage 3b went were reclassified as 3a (18.9% in subjects <70 years of age, 24.7% in women), much greater than the 3.1% found in our study. This may be due to the differences in population with nephro-urological disease (younger and with a higher proportion of men).
Esteve Poblador et al.20, with a study population with a mean age of 73.5±8.3 years, and Elorza-Ricart et al.21, found a similar overall incidence of eGFR <60 in the group ≥60, similar with both equations. In this last study, the overall percentage of reclassification from stage 3a to eGFR ≥60 of 21% was reduced to 5.7% in subjects over 70 years of age.
Our results coincide with the reviews done by Stevens et al. and Early et al.11,23 in which the differences between both equations for eGFR are less significant in elderly patients than in the younger population and the differences are greater as the eGFR values increase (Figure 1 and Figure 2).
The eGFR is important for detecting CKD, evaluating the severity and rate of progression and for starting proper management. The MDRD-4 IDMS equation was developed in individuals with CKD and a decrease in glomerular filtration and their greatest limitations are imprecision and systematic underestimation at high levels; in order to avoid these limitations, a new more reliable equation was sought that was as reliable as the MDRD-4 IDMS equation at values <60ml/min/1.73m2 and more reliable at elevated values with results that were consistent across age, sex and radial subgroups. The CKD-EPI equation improves the eGFR, especially at levels greater than 60ml/min/1.73m2, but limitations in precision persist. As the authors concluded,8 the CKD-EPI equation did not overcome the limitations of serum creatinine as a marker of endogenous filtration, which suggests that age, race and sex do not encompass all of the variability in the measurements not associated with eGFR from creatinine; the lower bias of the CKD-EPI equation versus the MDRD-4 IDMS equation would decrease false positive diagnoses for CKD but not in all subgroups, including those over 70 years of age.
The decrease in eGFR is an established risk factor for cardiovascular disease, death and end-stage kidney disease and is important to determine which formula best determines this risk. In the ARIC and AusDiab studies9,10, the CKD-EPI equation was associated with a better prognostic classification when compared with MDRD-4 IDMS in individuals of medium age for overall mortality, cardiovascular episode and end-stage kidney disease. In the CKDPC15 meta-analysis, improvement in reclassification was positive in the majority of subgroups defined by age (<65 and ≥65), sex, race/ethnicity (whites, Asians and blacks) and the presence or absence of DM and HTN. The results of the cohorts with CKD and high risk showed a high consistency with the general population, which is why one can conclude that CKD-EPI classifies fewer individuals with CKD and more reliably categorizes the risk of mortality and end-stage CKD than the MDRD equation in a wide range of populations.
The CKD-EPI equation was developed and validated in a population with a low percentage of women over 70 years of age and its results in this subgroup are scarce and controversial. In the 3C study,16 in subjects over 65 years of age, the estimation of excess risk associated with the decrease in eGFR was similar with the MDRD-4 IDMS and CKD-EPI equations. Conversely, in the NHANES study,24 the CKD-EPI equation improved the risk classification in individuals, especially in those over 65 years of age.
The strong points of the study are the creatinine measurements done in a single laboratory by a standardized method with a well-defined population and a large number of individuals over the age of 60. In addition, as this is a study performed in PC according to routine clinical practice, it allows for the true impact of the use of one eGFR formula or another to be evaluated.
No reference method for eGFR was used in our study. This may be considered a limitation, though the primary objective of the study was not to evaluate the precision of eGFR but rather to study the differences between both equations. Another limitation of the study is that the diagnosis of eGFR <60 was done using a single creatinine measurement, though this is common in epidemiological studies. Finally, the race variable was not taken into account, though Caucasian race predominates in our area and especially in this age group.
While there is currently no optimal formula for eGFR, the majority of studies note that CKD-EPI may be more useful for reducing false positives in the diagnosis of CKD and it has better prognostic ability for risk of death and end-stage kidney disease; its use in PC would avoid classifying healthy individuals as “sick” and reduce prescribing of medications to reduce the supposed increased cardiovascular risk, as well as allow for improved management of prospective risk in individuals with CKD. However, our study also indicates that there are no great differences between the two equations in individuals over 70 years of age. Follow-up in our cohort may provide new data in the future.
Acknowledgements
This study was financed by the Carlos III Institute/Healthcare Research Fund (PI11/02220).
Special thanks to IDIAP J Gol for their management of the project, and to Lola Valero, from the Technical Section at DAP Costa de Ponent, for extracting the data.
Conflicts of interest
The authors declare that they have no conflicts of interest related to the contents of this article.
Table 1. Global individual characteristics by sex.
Table 2. Glomerular filtration rate characteristics based on estimating methodology by sex, age and globally.
Table 3. Classification of the sample analysed, taking the MDRD4-IDMS method as a reference, between the different categories for estimating glomerular filtration rate according to CKD-EPI. Classification based on the revised KDIGO guideline. The results are shown
Table 4. Characteristics of the individuals by CKD-EPI stage, for individuals in stage 45-59 (3a) for MDRD-4 IDMS
Table 5. Characteristics of the individuals according to CKD-EPI stage, for individuals in stage 30-44 (3b) for MDRD-4 IDMS
Table 6. Prevalence of estimated glomerular filtration rate < 60 in different studies
Figure 1. Association between the glomerular filtration rate estimated with MDRD-4 IDMS and CKD-EPI by sex for individuals with MDRD-4 IDMS <90 and age
Figure 2. Association between the glomerular filtration rate estimated with MDRD4-IDMS and CKD-EPI by sex for individuals with MDRD-4 IDMS <90 and age 70 years.